Breakthrough Alzheimer’s drug produced in Switzerland
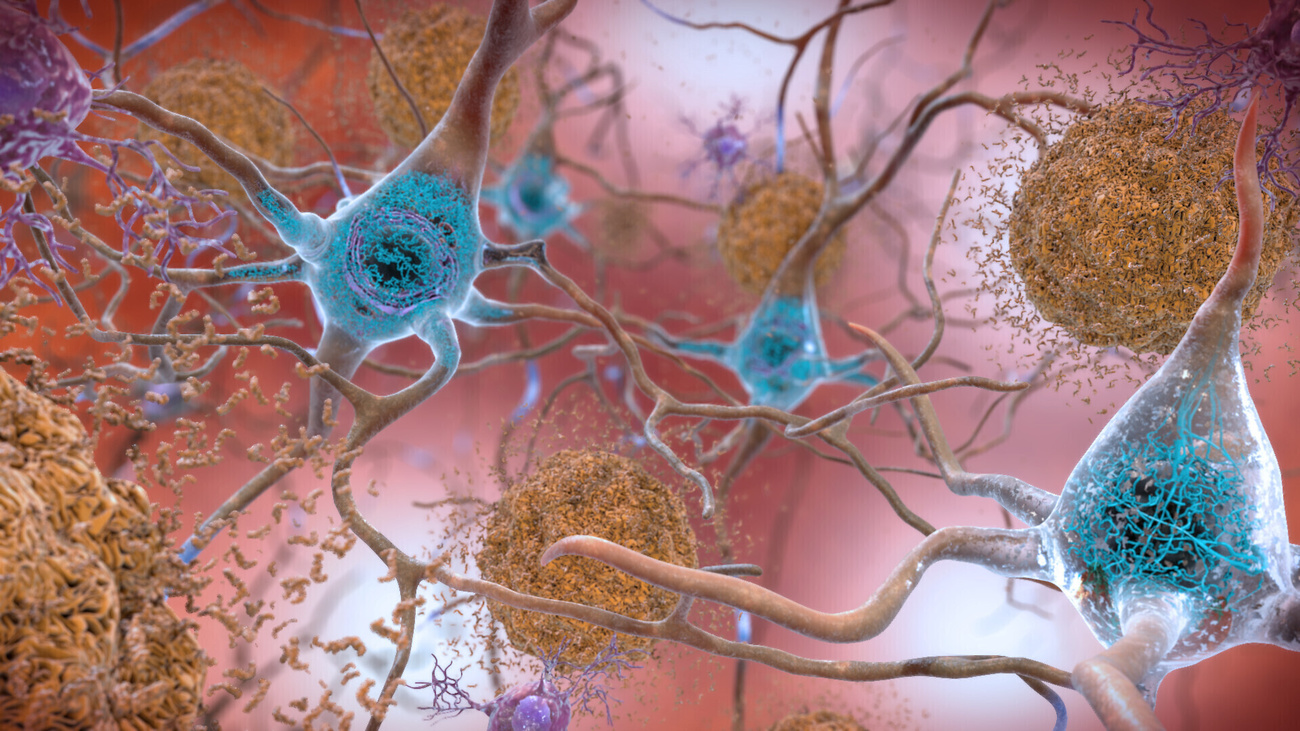
The US Food and Drug Administration gave the greenlight to Leqembi this week, the first drug for Alzheimer’s disease to receive full approval by US authorities in two decades. Biogen’s new factory in north-west Switzerland is the only producer of the drug’s active ingredient, lecanemab.
The town of Luterbach in canton Solothurn isn’t a frequent stop-over for people working in the biopharmaceutical industry. The closest major airport is in Basel, over 70 kilometres and two train connections away.
But for millions of people suffering from Alzheimer’s disease around the world, the town gained new significance on Thursday, when the US Food and Drug Administrated approved Leqembi, the first drug for the neurodegenerative disease to receive full approval by US authorities in two decades.
While the drug isn’t a cure, it has shown to slow cognitive decline in patients who are in early stages of the disease. Clinical trial data suggested that this could slow decline by some 27% over an 18-month period. Although the benefits may be modest the Leqembi approval is widely considered a milestone because it’s the only approved therapy that tackles the “underlying disease process” rather than simply the symptoms of the brain disorder, indicated the FDA in its approvalExternal link.
Luterbach, which at last count had just over 3,600 people, is the seat of the only production site globally of the active ingredient in Leqembi developed by the Japanese company Eisai and sold in partnership with US biotech firm Biogen.
What the town lacks as a pharmaceutical hub it makes up for with good infrastructure, a plot size big enough for a 36,000 square metre biologics factory (equivalent to around 4 football fields), and a plentiful water supply thanks to its proximity to two major rivers – the Aare and Emme.
Top universities and biotech hubs as well as the stable political and economic situation in Switzerland were also key factors, Tristan Schmitz, who heads Corporate Affairs at Biogen’s international headquarters in Baar, Switzerland told SWI.
Complex production process
Leqembi, which is administered by intravenous infusions every two weeks, is a monoclonal antibody that targets a protein called beta-amyloid that clump together to form plaques in the brains of people who have Alzheimer’s disease.
As a biological medicine, in contrast to a chemically synthesized drug, the process for producing the active ingredient lecanemab is complex and can take several weeks.

According to Schmitz, the Luterbach factory is responsible for four stages of the production process, starting with the cultivation of mammalian cells at a quantity large enough to manufacture the product. Then comes the fed-batch process whereby cells are fed at regular intervals with nutrients that they need for growth and the production process.
This is then followed by a series of purification steps, and by the formulation stage in which the product is converted into a stable form so it can be transported for final processing. Schmitz confirmed to SWI that Biogen’s facility in North Carolina will support with final production of the drug.
Biogen invested CHF1.5 billion ($1.6 billion) in the facility since it received the building permit in 2016. It launched operations in 2021 after Swiss medicines regulator Swissmedic granted the site “Good Manufacturing Practice” multi-product license. There are currently around 500 people working at the Luterbach site, many of which were recruited over the last couple of years.
The Luterbach factory is expected to triple Biogen’s biologics production, which currently includes several products for neurological diseases such as multiple sclerosis. Schmitz didn’t provide more detailed information on other drugs being produced at the facility. The factory intended to produce Biogen and Eisai’s first Alzheimer drug, Aduhelm, which received accelerated FDA approval in 2021 but never gained market traction due to questions about the cost-benefits of the drug and its price tag of $56,000 per year.
Long history
Biogen’s ties to Switzerland date back much farther than the Luterbach facility. The company, considered one of the pioneers in the biotech industry, was founded in Geneva in 1978 by several scientists, among them, Charles Weissman, a Swiss-Hungarian molecular biologist who received his medical degree and doctorate from the University of Zurich.
The company moved its headquarters to Cambridge, Massachusetts in 1982 but established its international headquarters in Baar, in canton Zug, where it employs around 400 people.
Whether Swissmedic, which received an application for approval in May, will also approve Leqembi and its price are still to be seen. US Medicare has agreed to cover 80% of the $26,500 annual price tag. A decision by Swissmedic is expected in autumn 2024.
Additional reporting by Tomoko Muth. Edited by Virginie Mangin.

In compliance with the JTI standards
More: SWI swissinfo.ch certified by the Journalism Trust Initiative











You can find an overview of ongoing debates with our journalists here . Please join us!
If you want to start a conversation about a topic raised in this article or want to report factual errors, email us at english@swissinfo.ch.