
Roche working ‘around the clock’ to provide coronavirus test
After getting the green light to deploy its coronavirus diagnostic test, Swiss pharmaceutical giant Roche says it is working around the clock to make it as available as possible.
Last Friday, the world received a glimmer of hope in the fight to contain the spread of Covid-19 that has left few countries unscathed. The US Food and Drug AdministrationExternal link granted Emergency Use Authorisation approval to the so-called “cobas SARS-CoV-2 Test”, making it the first commercial diagnostic test for the coronavirus.
With this designation and the similar CE-Mark for in-vitro diagnostics in Europe, the new test can be deployed in hospitals and laboratories in the US and Europe – offering the potential to massively scale up testing.
In contrast to existing tests, Roche CEO Severin Schwan told Swiss public television, SRF, that the system is highly automated, which will “reduce the burden on the healthcare system.”
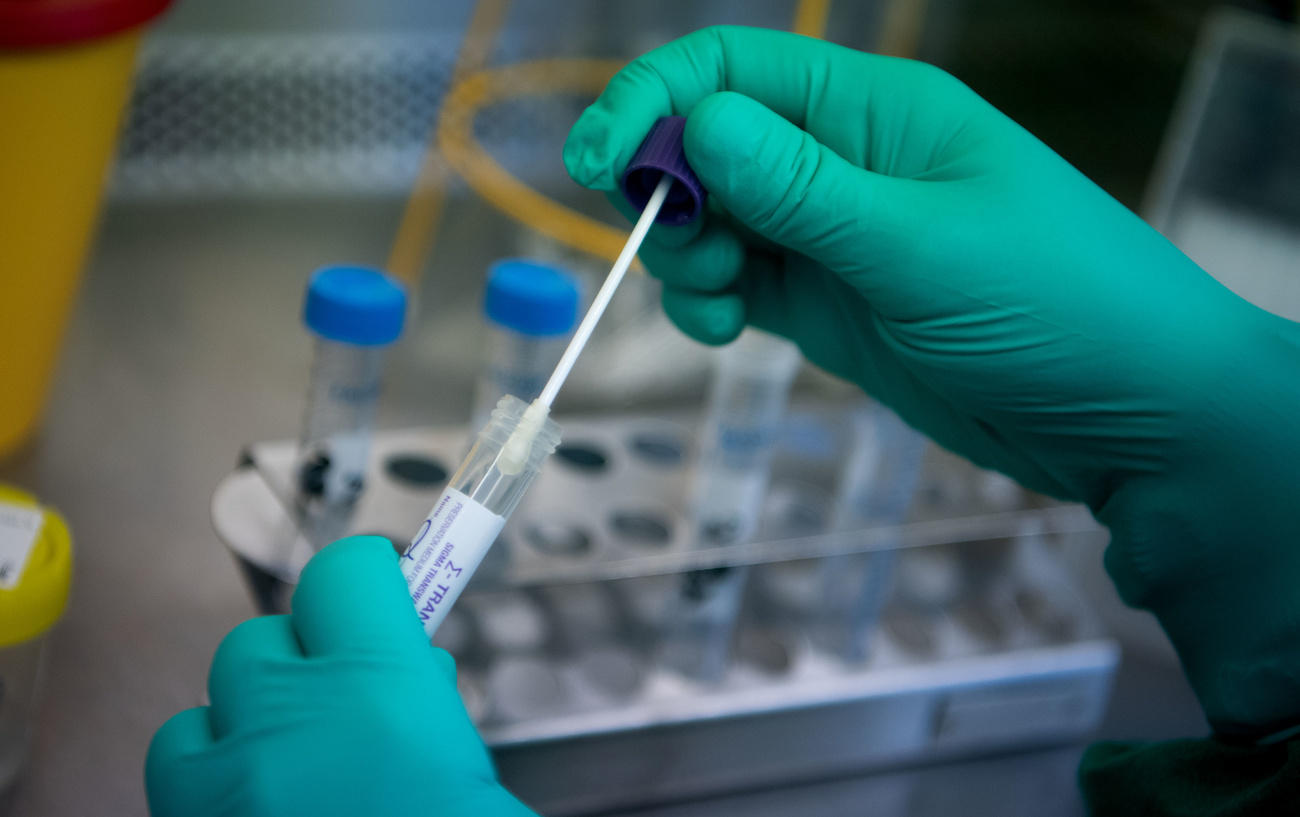
The test detects the genetic signature (RNA) of the SARS-CoV-2 using a nasal or throat swab. After a healthcare provider collects the sample from the patient, it is sent to a lab equipped with the test and the cobas 6800 or 8800 system, which is manufactured in Rotkreuz, less than an hour from Zurich.
Schwan said that the system can conduct more than 4,000 tests per day. Results are available in 3.5 hours, making it ten times faster than the company’s existing test.

More
Roche CEO explains new Covid-19 test
In a press statementExternal link, Roche wrote that their cobas 6800/8800 systems, which look like massive printers, are “widely available”. A Roche spokesperson declined to comment on how many there are, or where they are, exactly. The company expects to supply about 400,000 tests per week in the US and more than three million globally.
“Roche is committed to delivering as many tests as possible and is going to the limits of our production capacity,” the company wrote in a statementExternal link. According to a Roche spokesperson, the tests will be distributed based on where they can have the greatest patient and community impact.
The company has also refrained from commenting on the price, stating that “the priority is to make the tests available to those who need them”.

In compliance with the JTI standards
More: SWI swissinfo.ch certified by the Journalism Trust Initiative


























You can find an overview of ongoing debates with our journalists here . Please join us!
If you want to start a conversation about a topic raised in this article or want to report factual errors, email us at english@swissinfo.ch.