Swiss implant makers come under the microscope
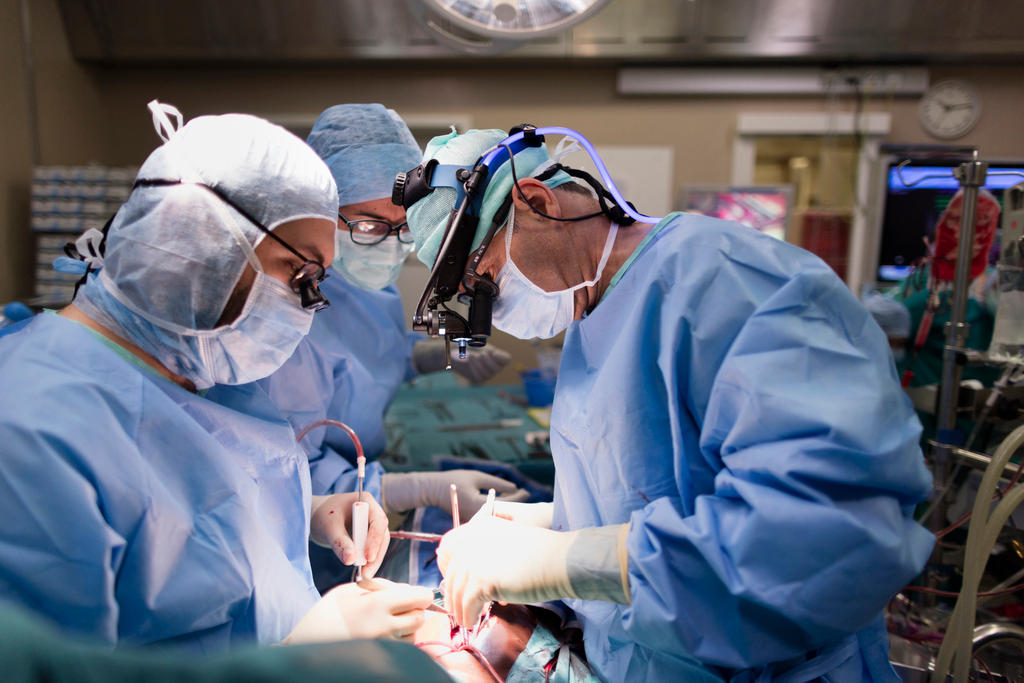
The Implant Files revelations of faulty implants and weak safety standards are raising alarm bells for Switzerland’s rapidly growing medical device industry. Manufacturers and industry bodies say that safety standards have improved in the last decade, but do they go far enough?
The market for implanted medical devices is booming globally. Tens of thousands of people are walking around with implants, from hip replacements to pacemakers and defibrillators.
According to the Implant FilesExternal link released on Monday, many of these people face serious health risks, in some cases fatal, from what are intended to be life-saving implants. The research was conducted by 250 journalists across more than 30 countries, including Swiss journalists from Tamedia, that are part of the International Consortium of Investigative Journalists (ICIJ).External link
The investigation suggests that products are often rushed onto the market with sub-standard testing or approval procedures, and for many, recalls are too late – the damage is irreparable. In Switzerland, there were approximately 1,400 complications due to medical implantsExternal link last year according to report on Swiss Public Radio, SRF. This includes, for example, spinal implants that disintegrate in the body causing long-term damage.
Post-scandal reforms
According to Peter Studer, Head of Regulatory Affairs at Swiss MedtechExternal link, which represents the interests of the Swiss medtech industry including implant makers, the journalist consortium report refers largely to cases that occurred before major changes in the medical device industry from 2012.
In a written statement to swissinfo.ch, he said that the Implant Files focus on cases around the time or prior to the infamous PIP scandal, when French company Poly Implant Prothèse was found to be illegally manufacturing and selling breast implants made from cheaper industrial-grade silicone since 2001.
+ More on how the PIP scandal affected Switzerland
In reaction to the PIP scandal, there was a major effort to reduce the number and improve the quality of notified bodies, which are organisations legally designated to assess the conformity of certain productsExternal link with laws before they go on the market.
There have also been efforts to improve the speed and accuracy of reporting of issues through the SIRIS Swiss Implant RegistryExternal link launched in 2012 and the reporting system of SwissmedicExternal link, the Swiss Agency for Therapeutic Products.
In an email to swissinfo.ch, Swissmedic said that in the case of serious events, authorities take the necessary measures – up to and including a recall or market withdrawal. Every recall and other “Field Safety Corrective Actions” (FSCA) reported to Swissmedic is published on a designated websiteExternal link.
Do the reforms go far enough?
But do these reforms go far enough in protecting patients? Swiss-based SonovaExternal link, one of the largest manufacturers of hearing care solutions, has confidence in its safety procedures. It is the parent company of Advanced BionicsExternal link that had a cochlear implant device recall prior to 2012 listed in the ICIJ database of 70,000 recalls, safety alerts and field safety notices.
In a statement to swissinfo.ch, Sonova explained that Advanced Bionics’ quality management system has been certified by globally recognised standards and its products are submitted for review and approval by the relevant regulatory agency before distribution. Any adverse events with products like its implantable cochlear stimulator system are reported to applicable regulatory agencies and made available to customers in its product reliability report.
Medical device industry
Medical devices (medtech is often used synonymously) includes instruments used for medical purposes that are not drugs and can range from implants and heart pacemakers to syringes, wheelchairs, and MRIs. The Swiss medtech industry generates annual sales of CHF15.8 billion ($15.9 billion) and exports of CHF11.3 billion according to Swiss Medtech. There are more than 1,400 medical device companies employing more than 58,500 people in Switzerland and the industry contributes 2.3% to Switzerland’s GDP.
Swiss Medtech reports that nearly 40,000 knee and hip prosthetic joints and 70,000 artificial lenses are implanted each year, while some 4,500 patients receive a pacemaker and an implantable defibrillator.
But the journalistic consortium’s report argues that “implants don’t require large-scale human trials for devices like they do for prescription drugs. As a result, authorities have allowed faulty devices to come onto the market”.
Swiss MedtechExternal link’s Studer argues that it is misplaced to compare medical devices to pharmaceuticals. He says that there are 10,000 medicinal products compared with 500,000 different medical devices that have huge variations. The range of applications for medical devices means that “authorisation procedures can’t be adapted one to one to medical devices compliance procedures”.
However, in many of the cases reported by the journalists, the problems didn’t show up until long after a device was implanted and many devices showed no signs of problems when tested on animals, indicating that more robust testing could have prevented significant pain and suffering.
Other concerns in the testing and approval system have also been raised by Swiss parliamentariansExternal link in recent days including the fact that the onus is often on doctors and hospitals to report problemsExternal link, which doesn’t always happen, said Bernhard Bichsel, Head of Medical Devices at Swissmedic in another report on Swiss public broadcaster, RTSExternal link.
In the fast lane
Medtech (or medical devices) is one of the fastest growing industries in Switzerland with more than 4,000 new employees in the industry in the last two years. There are more than 1,400 medtech companies, which includes implant manufacturers, employing more than 58,500 people in Switzerland.
Aside from some of the big players like Johnson & Johnson Medical, Roche Diagnostics and Sonova, the large majority are start-ups. Some 93% of companies employ fewer than 250 employees and four out of five companies have fewer than 50 employees, according to the 2018 sector studyExternal link by Swiss Medtech
This raises the question that has been a thorn in the side of the pharmaceutical industry for decades. How do you balance the need for robust safety regulations with the desire to get potentially life-saving products on the market as quickly as possible?
For small companies with limited bandwidth, this question is even more pressing. The sector study found that many medtech companies were struggling with the increasing requirements for quality and documentation due to new European Union regulations (see box below).
Medical device regulations in Switzerland
The Swiss Medical Devices OrdinanceExternal link has been in force since 2002 and was updated in 2010 to harmonise Swiss law with the EU’s revised medical devices directives. Switzerland and the EU have established a mutual recognition agreement which means that the country is integrated into the European market surveillance and that equivalent relevant legal requirements to the EU exist in Switzerland.
In spring 2017 two new regulations were adopted by the European Commission on medical devices (MDR) and in-vitro diagnostics (IVDR) with the aim of significantly improving patient safety by improving risk assessment, market surveillance and increased transparency. It will become mandatory in EU member states starting from May 2020 and May 2022, respectively.
Discussions are currently underway in the Swiss government regarding a revised Medical Devices Law to align with the latest EU regulations.

In compliance with the JTI standards
More: SWI swissinfo.ch certified by the Journalism Trust Initiative










You can find an overview of ongoing debates with our journalists here . Please join us!
If you want to start a conversation about a topic raised in this article or want to report factual errors, email us at english@swissinfo.ch.