How can a drug cost $2.1 million?
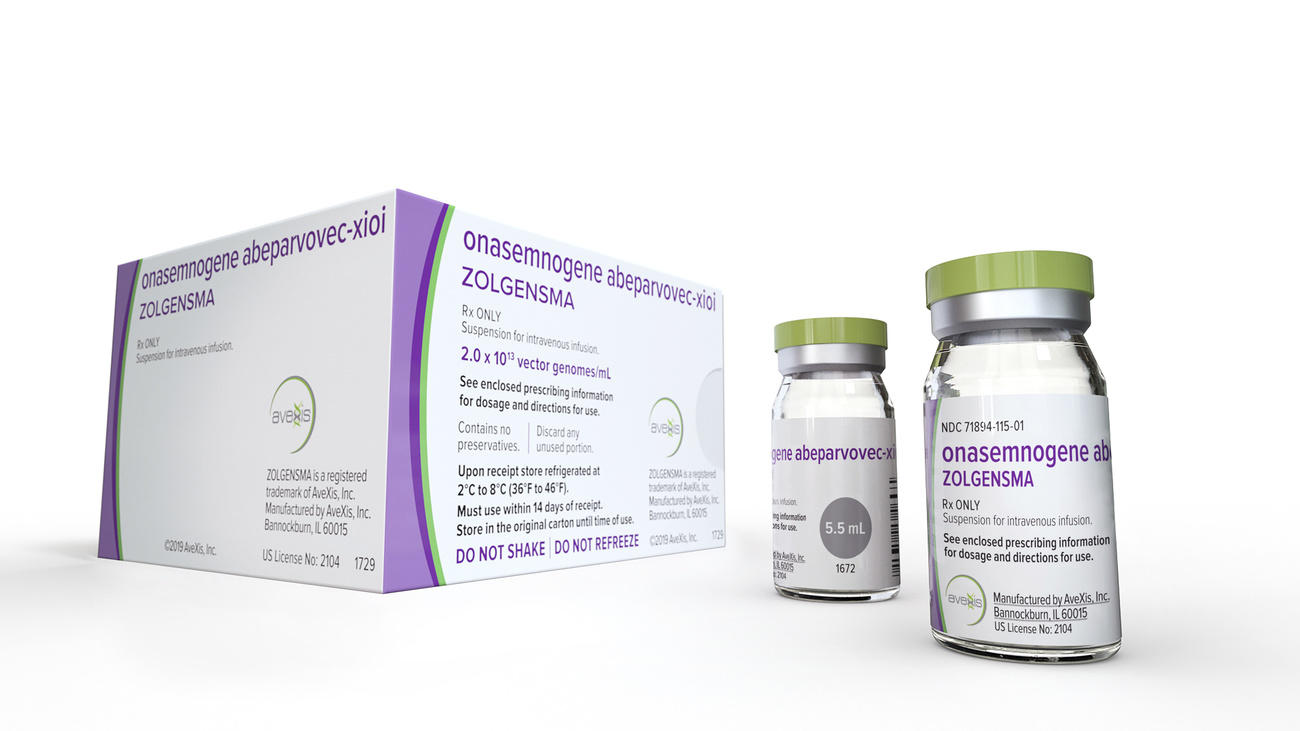
US authorities have approved a $2.1 million (CHF2.1 million) gene therapy from Swiss-based Novartis to treat a deadly childhood disease, making it the most expensive drug ever. Such a price tag has also generated criticism. Can it be justified?
The recently approved drug ZolgensmaExternal link is a gene therapy, which is a relatively new but rapidly growing field of drug development whereby a correct copy of a gene replaces mutated genetic material that is causing a disease. In a sense, these therapies address the root cause of a disease – a defective gene – and in doing so could eliminate a host of deadly childhood diseases, rare blood disorders and hereditary cancers.
Zolgensma treats spinal muscular atrophyExternal link, a deadly disease caused by a genetic defect that weakens a person’s muscles so dramatically that they become unable to move and can eventually impair the ability to swallow or breathe. Babies born with the acute form of the disease often die before the age of two. About one in 10,000 newborns is affected by the disease, making it one of the more common rare diseases.
Zolgensma is the 17th cell and gene treatmentExternal link to make it on the US Food and Drug Administration’s approved list. But the FDA expects by 2025 to be approving ten to 20 cell and gene therapies a year. Some 2,600 gene therapy clinical trialsExternal link in 38 countries are underway or have been completed.
Swiss pharmaceutical companies Novartis and Roche are at the forefront of the gene therapy revolution, with recent acquisitions of some smaller biotech firms.
Zolgensma is not the only treatment to raise eyebrows because of its price tag. A couple of years ago, a debate ensued in Switzerland over the $60,000 price tag for a drug to treat hepatitis C.
In a commentaryExternal link on CNBC, Novartis CEO Vas Narasimhan argued that gene therapies represent a medical breakthrough in the way that they offer hope of a cure for deadly genetic diseases with a single dose. In some cases, the alternative is a multi-dose treatment with incremental improvements.
Zolgensma, approved for children under the age of two, is a one-time infusion that takes about an hour. Roche is also developing an oral solution to the disease.
The alternative is the current treatment regimen under the brand name SpinrazaExternal link that is taken four times a year for life. The list price is $750,000 for the first year and then $350,000 per year after that, so about $4 million a decade.
The independent non-profit group Institute for Clinical and Economic Review, which rates the value of expensive new medicinesExternal link, calculated that the price of the new gene therapyExternal link is justifiable at a cost of $1.2 million to $2.1 million because it “dramatically transforms the lives of families affected by this devastating disease”.
The calculationExternal link is largely based on costs per year of life gained comparing the clinical trial outcomes for treatment options.
There are several. The first is how such a price is determined and what margins companies are making – especially given how little is known about the costs of developing such drugs. Novartis didn’t develop Zolgensma but acquired it through the $8.7 billion purchaseExternal link of US firm AveXis.
In addition, many companies argue that prices are calculated using a value-based pricing model, which raises a host of thorny questions about how much a life is worth. There is also a dearth of evidence on whether the treatments are effective and potential risks, especially long-term. Some therapies on the market, such as Imlygic, haven’t lived up to their potential.
This is complicated by the fact that many gene therapies tackle rare diseases – in that they have the potential to improve dramatically the lives of a few people. There is some criticismExternal link that this is driving focus away from more common diseases like diabetes and hypertension.
There are also ethical issuesExternal link related to manipulating genes and fears of a slippery slope leading to designer babiesExternal link.
Ultimately though, the main source of controversy is how society is going to pay for these expensive treatments. Most health insurance systems are not designed for one-time treatments at such a price. Novartis has already said it is working with insurers to allow payments over five years, at $425,000 per year, and will give partial rebates if the treatment doesn’t work. But it remains unclear how this will work in each country.
A previous version of this article indicated that Novartis had purchased the firm AveXis for $8.7 million. The correct purchase price is $8.7 billion. We regret the error.

In compliance with the JTI standards
More: SWI swissinfo.ch certified by the Journalism Trust Initiative










You can find an overview of ongoing debates with our journalists here . Please join us!
If you want to start a conversation about a topic raised in this article or want to report factual errors, email us at english@swissinfo.ch.