Drug companies face vaccine dilemma

Pharmaceutical companies worldwide are poised for a race to find a vaccine for swine flu but are still waiting for the go-ahead from the global health authorities.
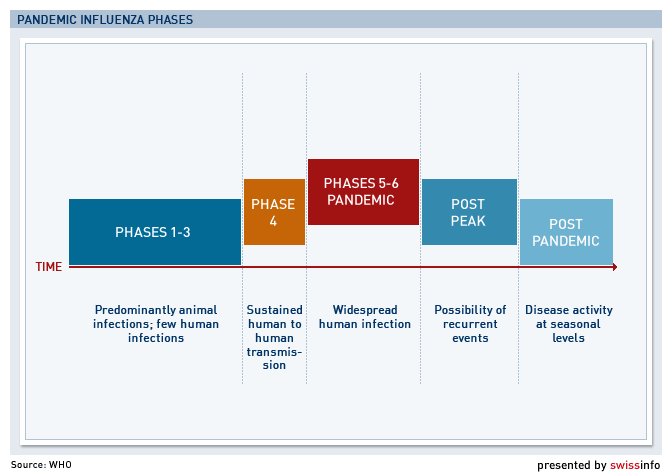
The World Health Organization (WHO) on Wednesday declared the virus had reached the pandemic Phase 5 outbreak stage – the second highest on its scale – but stopped short of officially calling for a vaccine, only saying talks had begun with manufacturers.
“It really is all of humanity that is under threat during a pandemic. We do not have all the answers right now but we will get them,” said WHO director-general Margaret Chan.
“I have reached out to companies manufacturing antiviral drugs to assess capacity and all options for ramping up production. I have also reached out to influenza vaccine manufacturers that can contribute to the production of a pandemic vaccine.”
The WHO informs manufacturers which vaccines – seasonal or pandemic – are required on a global level. Now health officials must decide whether to push pharmaceutical firms for a vaccine to stop the spread of swine flu – and thereby possibly divert drug makers’ resources away from regular seasonal flu vaccines – or to hold off to see how the virus, termed A/H1N1, evolves.
Seasonal flu kills up to 500,000 people worldwide annually. Chan noted on Wednesday that there was not yet enough evidence to stop production of the seasonal influenza vaccine.
“It’s a very big decision,” added WHO spokesman Gregory Hartl. “It means no one in the next flu season will get their seasonal flu shots. We need to weigh the pros and cons.”
According to the WHO, Phase 5 means there is a sustained human-to-human spread of the virus in at least two countries and that a pandemic could be imminent.
Producing sufficient vaccines for a pandemic would put a strain on the capacity of drug manufacturers, who produce around 780 million flu vaccine doses annually.
The severity of the swine flu remains unclear. The WHO has stressed that several infected people have already been cured and says it does not know how the virus will evolve. The way it is spreading and causes of death also need to be analysed.
Time to incubate
It could take four months for a vaccine to become commercially available. In production, versions of the virus are injected into the embryos of chicken eggs which are then incubated for several months. The egg whites are then extracted and blended into a vaccine. Swiss firm Novartis is one of the few manufacturers to have started to use the faster technology of cell cultures.
The first steps towards a vaccine are being taken by the United States Centers for Disease Control and Prevention (CDCP), where scientists are studying the biological makeup of the virus and have created the basic components for a vaccine.
The WHO and the CDCP will decide if a vaccine is required and at which point the basic elements, or a seed virus, would be passed onto pharmaceutical companies, which in turn would carry out tests in humans before producing the vaccine.
At least 20 companies make flu vaccines. A number have said they are in talks with the WHO and the CDCP about developing a vaccine and to discuss their capacity issues.
One possibility being considered by health officials is a combined shot for swine and seasonal flu.
There is also a risk of moving too fast in developing a vaccine. The US vaccinated 40 million Americans in 1976 after several cases developed of a strain of swine flu. But the epidemic never materialised and instead the vaccine is thought to have caused side effects such as neurological disorders in around 500 people.
“Way forward”
Novartis, one of the companies contacted by the WHO, said on Thursday it was keen to begin work.
“We have received the genetic code of the new virus strain in question and have started to evaluate production of a vaccine. We hope to receive the actual virus in our laboratories in the near future,” said a spokesman.
Novartis also announced that it was using data from a study on its pre-pandemic bird flu vaccine Aflunov to help develop the swine flu vaccine. In particular the use of an additive called MF59 that may help boost the effectiveness of a vaccine.
“The findings published about the H5N1 [avian flu] vaccine suggest a way forward for the present swine flu outbreak,” said Rino Rappuoli, head of research at Novartis Vaccines.
Dynavax Technologies and Lipoxen also presented research they said could help in developing a vaccine.
European Union health ministers held an emergency meeting on Thursday to look at how to prepare the bloc for the potential spread of swine flu.
“When a vaccine is available, we need to have a clear vision how it’s going to be rolled out across Europe,” said Robert Madelin, from the European Commission’s health department.
In the meantime, the WHO has asked countries to inform their populations about prevention, boost their lab capacity to be able to quickly test patients suspected to have the flu, and improve disease surveillance.
swissinfo with agencies
This swine flu variation, termed H1N1, is an influenza A virus spreading from person to person. It contains DNA from avian, swine and human viruses. Although swine flu viruses normally infect only pigs, they do sometimes cross the species barrier to cause disease in humans, according to the WHO.
It is passed from human to human by sneezing, coughing or by hand contact. The WHO says swine flu is not transmitted to people eating properly handled and prepared pork or other products derived from pigs. The virus is killed in cooking temperatures above 70°C.
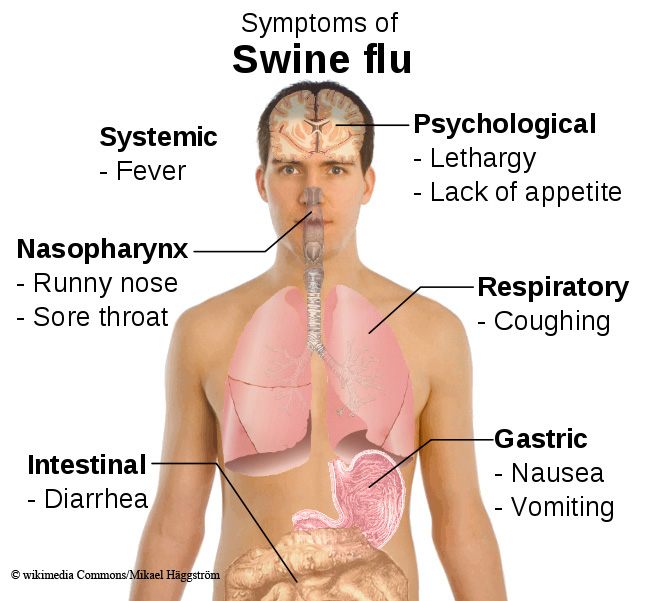
Swine flu is characterised by common flu symptoms – sudden fever, muscle aches, sore throat and dry cough – but may cause more severe vomiting and diarrhoea.
New flu strains can spread fast because no one has natural immunity and a vaccine can take months to develop.
To protect from infection:
Avoid close contact with people who appear unwell and who have fever and cough.
Wash your hands with soap and water frequently and thoroughly.
Practise good health habits including adequate sleep, eating nutritious food and keeping physically active.

In compliance with the JTI standards
More: SWI swissinfo.ch certified by the Journalism Trust Initiative











You can find an overview of ongoing debates with our journalists here . Please join us!
If you want to start a conversation about a topic raised in this article or want to report factual errors, email us at english@swissinfo.ch.