Big Pharma steps up race for AI-discovered drugs

Pharmaceutical giants, including Swiss firms Roche and Novartis, are betting big on artificial intelligence to discover new drugs to treat a range of diseases. But there’s a long road ahead to bring AI-discovered drugs to patients.
Matthias Steger’s discovery of the drug candidate EA-2353 for retinitis pigmentosa – a rare, degenerative eye disease – started very low tech: with a notebook and a pencil.
Check out our selection of newsletters. Subscribe here.
For nearly a decade, Steger, a trained medicinal chemist, jotted down chemical structures that researchers found had an impact on stem and progenitor cells – those that can regenerate in damaged tissue. But to arrive at a drug candidate, Steger, who spent 10 years in drug discovery and other roles at Roche before becoming an entrepreneur, needed to find the pattern in the chemical structures. This would take years and a lot of money testing in a lab, and even then, a lot would be left to chance.
“Discovering a new molecule is like finding a needle in a haystack,” Steger told SWI swissinfo.ch. “Even for a trained chemist, there’s significant guesswork.” It takes on average a decade and some $2.5 billion (CHF2.3 billion) to bring a new drug to market.
In hopes of expediting the process, he sent the chemical structures to Gisbert Schneider, a former Roche colleague who now teaches computer-assisted drug design at the Swiss federal institute of technology ETH Zurich. Schneider used his AI models to identify molecules that had the desired biological activity based on the patterns in the chemical structures. Steger and his colleagues tested and synthesised the molecules over several years to generate two drug candidates. One of which is EA-2353, now in early-stage clinical trials.
“I’m not sure we would have been able to find the drug candidate without AI,” said Steger, who with Schneider founded start-up Endogena in 2016, with offices in Zurich and San Francisco, to further develop the two drug candidates. “Algorithms can see patterns that aren’t visible to the human eye.”
AI’s potential to discover new drug candidates, in a fraction of the time and cost as traditional approaches, has fueled an AI investment boom. Over the past decade investors have pumped more than $18 billion into some 200 “AI-first” biotech firms and start-ups, those where AI is central to their drug discovery workflow, according to a Boston Consulting Group studyExternal link published last year.
These start-ups and tech firms have been at the forefront of the technology but, as AI-discovered drugs move into testing on humans, more big pharmaceutical companies, including Swiss giants Roche and Novartis, are jostling to get out ahead of peers.
Last year, Roche announced a multi-year research collaboration with US chipmaker Nvidia, one of at least eight AI deals the company has signed since 2019. In January, Swiss pharma firm Novartis offered Google DeepMind’s offshoot Isomorphic Labs $37.5 million upfront and another $1.2 billion if it achieves certain milestones developing three new drug candidates. These are a handful of more than 100 deals between pharma and AI drug discovery start-ups in the last decade.

More
How are you? What is on your mind? Take part in our big survey
Jumping on the AI train
Large pharmaceutical labs have been using computers to assist drug development for decades but until recently there was some reticence to rely too heavily on AI.
“After experimenting with artificial neural networks years ago, there was a feeling in pharma companies in the 2000s that AI hadn’t delivered on its promise,” said Schneider. These early networksExternal link or AI algorithms lacked the sophistication and data along with powerful machines to perform massive calculations.
Today, the mindset has changed. “There’s now much more willingness to accept recommendations made by an AI algorithm, and no pharma company wants to be left behind.”
Artificial Intelligence refers to a range of advanced computational and modelling techniques that analyse and learn from often large and complex data sources and can generate insights or perform tasks that would typically require human-level intelligence, at a scale and speed beyond human capability. Deep learning, which harnesses artificial neural networks to acquire knowledge from data, is a common AI technology used in drug discovery. Source: Boston Consulting Group
Behind the shift are recent advances in deep learning, generative AI tools like ChatGPT, computing power, and knowledge about genetics and molecular biology.
The latest generation of AI models can analyse and find patterns in vast and disparate datasets and even images, making it extremely useful for drug discovery where scientists are dealing with trillions of cells and around 20,000 protein-coding genes in any one person.
In 2020, Google’s AI research subsidiary DeepMind launched AlphaFold, an AI algorithm which can now predict the three-dimensional structures and interactions of proteins, RNA and DNA. This was instrumental in determining the protein structures of SARS-CoV-2, helping scientists develop Covid vaccines in record time.
AlphaFold has not only fueled research into a host of new drug targets but it confirmed AI’s potential for scientific breakthroughs. There are now a host of proprietary and open-source AI software tools that are being deployed in pharma companies to search medical journals for relevant data, screen molecule libraries for promising drug candidates and identify disease targets. Some studiesExternal link suggest AI could reduce the time and cost of drug discovery by 25-50%.
+ Artificial intelligence brings about record-fast vaccines
“AI, including machine learning and large language models, isn’t completely new technology,” says Elif Ozkirimli, who heads computational science products for research and development (R&D) at Roche in Basel. “But the adoption and scale have tremendously accelerated in the past two years.”
Roche has been investing around $3 billion a year to overhaul the company’s digital infrastructure and make AI a more integrated part of its R&D process according to a recent investor presentationExternal link. A few years ago, Roche hired top computational biologists from MIT and Cambridge University to build out a team of some 400 people in the computational sciences department in its San Francisco subsidiary Genentech alone. Hundreds more work in Basel and other sites.
In 2021, the company bought Prescient Design, a three-person New York start-up, to create a suite of algorithms trained on both public data and Roche’s own proprietary data from experiments and clinical trials. These have already helped find new disease indications for older drugs and prioritise drug candidates that have the highest chances of success.
From search to generate
Beyond making drug discovery more efficient, AI has the potential to identify and even generate molecules that chemists haven’t even dreamed of. Some algorithms, like the one Schneider used for Endogena, are even generating molecules from scratch.
“Instead of looking for drugs by screening molecules one after the other, generative AI is inverting the drug discovery process. It allows us to design molecules with certain properties instead of searching for them,” Schneider told SWI swissinfo.ch.
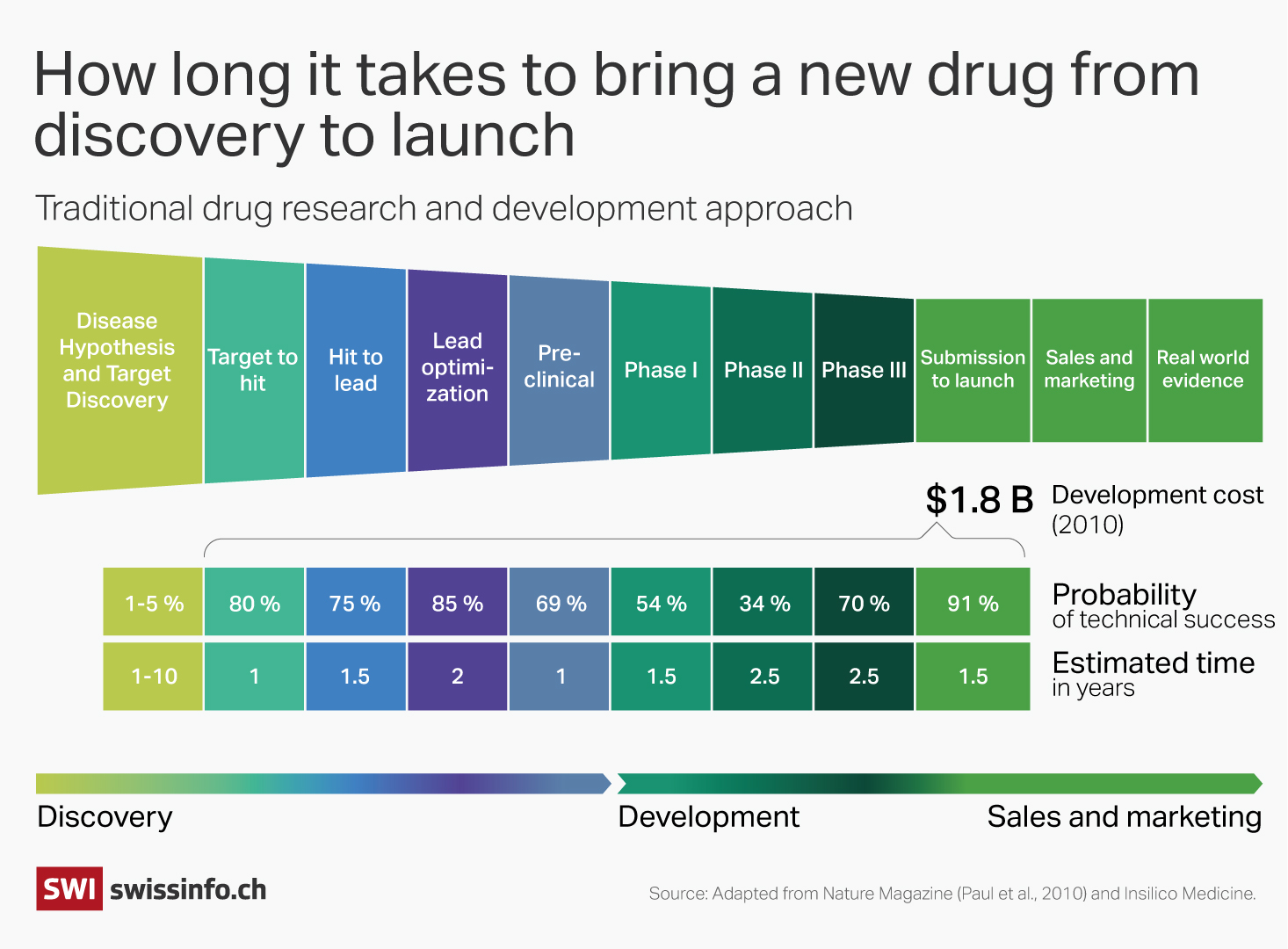
Several start-ups are already doing this. Hong Kong-based start-up Insilico used AI models to identify the drug target (the molecule associated with a disease) and create a molecular structure for pulmonary fibrosis – a serious lung disease. The drug candidate, which is now in phase II trials, was discovered in 18 months and at a cost of $3 million, far less than through traditional approaches. In 2022, Insilico signed a deal with French pharma giant Sanofi, worth up to $1.2 billion.
Genentech chemists and computational scientists also developed an AI model, GNEProp, to identify small molecule antibiotics for “superbugs” – bacteria that has become resistant to antibiotics. The model, which was trained on data on the antibiotic activity of two million small molecules, is used to predict molecules that would be active against harmful bacteria. Some of the molecules produced by the deep learning algorithm have completely different structures than those used to train it.
The scientists tested some molecules in the lab and found a 60-fold better hit rate (a positive result that the molecule has the desired biological activity) for the AI predictions than it found through its own experiments in 2017. The company is now taking some of the molecules into pre-clinical stages while retraining the model on the lab findings to make more accurate predictions in the future.
+ Roche takes on the tech sector
“Drug discovery is a little bit trial and error,” said Ozkirimli. “With AI, we are trying to incorporate some of those trial and errors into machine learning models so they can make better predictions.”
Marathon not a sprint
Despite the massive investment and excitement over novel discoveries, there’s still some reticence to boast about what AI has actually achieved. Neither Roche nor Novartis share names of any AI-discovered drugs in clinical trials.
Success on a computer screen or even in a lab doesn’t always predict success in patients. Drug development has a miserable failure rate: some nine out of ten drugs discovered through traditional methods fail in clinical trials, when drugs’ safety and efficacy are tested in humans.
It’s too early to say whether AI-powered drugs will have more luck, and if so, which AI algorithms behind them make the most accurate predictions.
+ Switzerland, land of pharma, tries to reel in AI start-ups
“There is still a lot we don’t know about human biology, the evolution of disease and why some patients respond better to drugs than others,” Schneider at ETH Zurich told SWI. “There’s a tendency now to overhype the potential benefits of AI tools, because we forget this element of chaos when we interact with human biology.”
According to a studyExternal link published in April, there have been at least 75 drug candidates in clinical trials in the last decade that were developed by firms where AI is central to their drug discovery. Some 80-90% of the candidates that went through phase I clinical testing were successful. This is higher than the industry average of 50-60%.
However, some expertsExternal link criticised the study methodology, arguing that the extent AI was used to design these drugs varied considerably, making it hard to generalise about AI’s success. Moreover, phase II, when drugs are tested for efficacy in a larger patient cohort, is considered a greater determinant of success.
Some drug candidates have already faced setbacks. Last October, one start-up Exscientia announced it was winding down an early clinical trial study of its AI-based cancer drug candidate EXS-21546. This came a few months after another drug by London-based BenevolentAI reported lower efficacy in early trials of its AI-designed drug than hoped.

More
AI could ‘add billions’ to Swiss company profits
“Most drugs fail not because there’s something wrong with the molecule. In many cases, the molecule does exactly what it should be doing,” said Steger. “It’s actually that the link between the molecular biology and the patient’s own pathology doesn’t pan out as the hypothesis predicted.”
Even if AI-generated drugs fail in clinical trials, researchers hope that this information is fed back into the models to generate better drug candidates the next time around. The hope says Schneider is that companies “fail less and faster”, avoiding huge cost outlays and unnecessary testing of drugs in animals and humans.
“With the current generation of machine learning tools, I don’t think the 90% failure rate will dramatically improve in the near future. It may drop to 70%,” said Yaroslav Nikolaev, Chief Technology Officer at the Swiss start-up InterAx, which is using mathematical models and advanced biology assays for AI in drug development. “The real transformation in drug development is coming but we need more quality data.”
Endogena’s lead drug candidate should report initial results for its first trial this year. Then the pivotal study in a large patient cohort starts. Preliminary results for EA-2353 look promising, according to Steger. “To the extent that we used it, AI did its job,” Steger told SWI. “But it takes more than AI to make a successful drug.”
Edited by Virginie Mangin/ds
Correction: This article was updated on June 11, 2024 to clarify that there are 20,000 protein-encoding genes and that phase II clinical trials is specifically for testing efficacy.

In compliance with the JTI standards
More: SWI swissinfo.ch certified by the Journalism Trust Initiative













You can find an overview of ongoing debates with our journalists here . Please join us!
If you want to start a conversation about a topic raised in this article or want to report factual errors, email us at english@swissinfo.ch.