Revolutionising biomedical science: mini-brains in a vat

Miniature organs and cancers grown in the lab could give doctors and pharmaceutical companies the chance to identify the most effective drugs and therapies, before they are given to patients.
At the Innovation Park of the Swiss Federal Institute of Technology in Lausanne (EPFL), biomedical engineer and entrepreneur Colin Sanctuary places several small vials onto a table, each labelled with an organ of the human body: pancreas, skin, lung.
“Organoid technology in general isn’t new – it’s been around since the 1980s,” he tells swissinfo.ch. “But our technology is unique because it is the only synthetic gel system that allows the growth of human cells in an in vitro environment.”
Sanctuary is the CEO of QGelExternal link, a start-up based at EPFL that has developed the first synthetic medium for growing “organoids” – cultured human cells and tissues that resemble miniature organs in structure and function – in an industrial setting.
While organoids don’t actually look like miniature body parts from the outside, they behave remarkably like the brains, livers, kidneys, and intestines they are derived from.
And with their potential for testing drugs and modelling cancers, they could revolutionise biomedical science and personalised medicine.
Mini-tumours
Sanctuary explains that until now, the critical material for growing organoids in vitro has been a gel matrix derived from animals with cancer. But animal-derived gels are tricky to handle and vary from batch to batch, so it’s hard to apply the data that come out of these experiments to industrial settings.
Each vial on the table at the QGel facility contains a unique gel matrix formula. Once a patient’s biopsied cells – from an organ or a cancerous tumour, for example – have been added to the gel, they will grow into organoids that behave as they would in their natural environment: the human body.
The biological, physical, and chemical properties of each gel can be fine-tuned to accommodate different types of cells and applications. For example, a firmer gel can be produced to grow cartilage cells, while a softer gel would better suit cells taken from human eye tissue.
“We can manufacture a library of 100 different gel types, grow the same cancer in 100 different gels, and decide what gel type works best,” Sanctuary explains. “Once we have identified a gel that works for prostate cancer, for example, we can then mass produce it.”
The following video provides an animation of how QGel uses organoids to test different drugs.
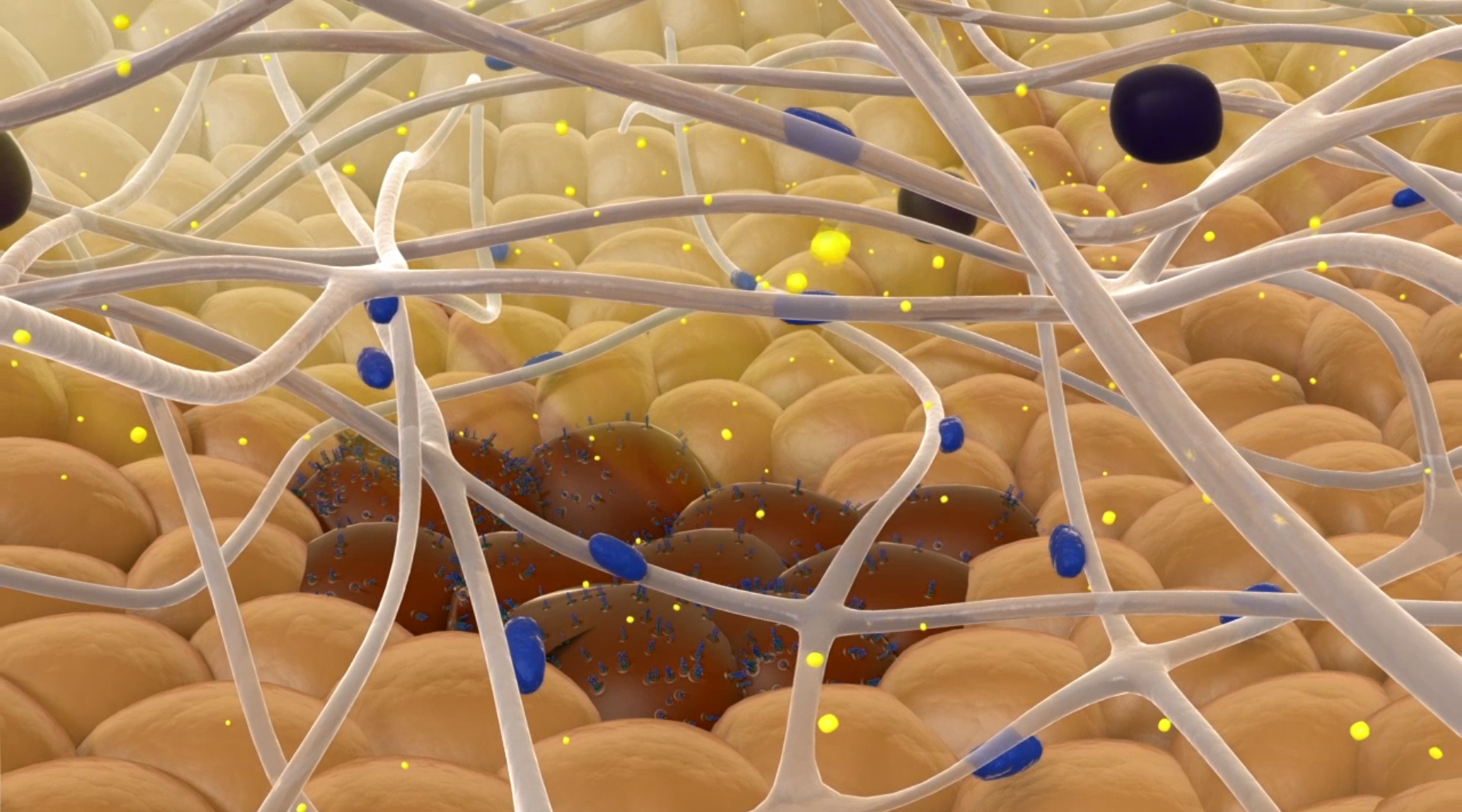
More
QGel drug screening animation
QGel’s clients include academic institutions doing basic research, but most are pharmaceutical companies, which use the gels to test their drugs on organoids before deciding which ones to optimise.
Their next goal is to make QGel’s technology available to hospitals and clinics for personalised cancer treatment. This is challenging from a regulatory perspective, but also because there will be no room for error or delay.
“With point-of-care treatment, you are working against the clock of the patient’s life to find that treatment, so there is a significant burden of proof,” says Sanctuary.
Repair and replace
Cancer can occur when a cell’s regulatory pathways – division, growth, and death – go wrong. So understanding those pathways, and what happens to them when they are affected by a genetic mutation, is another key application of organoid technology.
In Gerald Schwank’s laboratoryExternal link at ETH Zurich’s Department of Biology Molecular Health Sciences, researchers are using organoids to study exactly how certain genetic mutations lead to cancer.
To produce their organoids, Schwank and his team focus on culturing intestinal adult stem cells.
“We already know relatively well which mutations cause cancer,” Schwank told swissinfo.ch.
“What we don’t yet know is the functional impact of these mutations – in other words, how the mutations result in cancer. That’s why we take biopsies from healthy patients, establish organoid cultures, and introduce mutations in vitro that we know cause cancer. Then we can later on use those cultures as models for drug screening.”
In addition to exploring how disruption of cell regulatory pathways by mutations causes cancer, Schwank and his team are studying how to set defective cellular processes back on track.
They hope that ultimately, their research will allow them to use gene editing in organoid cultures to repair adult somatic stem cells from patients with certain genetic diseases, and then transplant the repaired cells back into the patients’ bodies.
“For example, imagine you have a patient with a genetic disease in the liver such as urea cycle disorderExternal link – there is currently no cure, and the life expectancy of patients can be severely decreased. The idea is that one could establish an organoid line from the liver of such a patient, and then use [a gene editing technology like] CRISPR/Cas9External link to correct the mutation, and then transplant the organoid back to the patient”, says Schwank.
What about the animals?
Research and industry efforts to make complex organoid technology more accessible to hospitals and pharmaceutical companies could potentially provide an alternative to using animals as in vivo models, test subjects, or even incubators for transplant organs.
But, Sanctuary explains, there are significant hurdles to replacing animal models with in vitro ones.
“Even though in vitro models are getting more and more relevant, in vivo models are still essential, and will be for the foreseeable future. The advantage is that testing on living beings take into account complex functions that an in vitro system just cannot capture, at least not today,” he says.
Nevertheless, organoids offer some significant advantages over animal models. Apart from the ethical and regulatory problems associated with animal research and testing, organoid models are more reliable and reproducible.
“Animal models have been used in the past by many researchers to study diseases, but of course, there is quite a difference between a mouse and a human,” says Schwank. “The problem with using animal models for transplantation research, for example, is that there is going to be a lot of variation, and a lot of factors you cannot control”.
Another main disadvantage that animal models are very expensive, and take up to a year to establish, whereas in vitro organoids can be grown in a month or two.
The tissues of the different organs in the human body – liver, blood, lungs, etc. – are all made up of cells. Most are differentiated, meaning they have developed into specialised cells that fulfil a certain function within that tissue.
However, a very small number of cells are undifferentiated, meaning that they can divide repeatedly to produce cells specialised to perform different functions within their organ of origin. These undifferentiated cells are called adult, or somatic stem cellsExternal link, and they are important for organ growth and repair throughout our lives. But when somatic stem cell division and growth processes go awry, cancer can result.
Embryonic stem cells are another type of stem cell that are derived from in vitro embryos, rather than from the organs of an adult. Embryonic stem cells are pluripotent, which means they can differentiate into the specialised cells of any bodily organ or system. The study of human embryonic stem cells for medical research has been a source of ethical debate, because using them involves manipulating and/or destroying in vitro human embryos.
In 2006, Japanese researchers discovered how to produce pluripotency in mature adult/somatic stem cells – these are known as induced pluripotent stem cells. The discovery earned lead researcher Shinya Yamanaka of Kyoto University a Nobel PrizeExternal link. In early 2016, researchers at Johns Hopkins UniversityExternal link used pluripotent stem cell technology to create “mini-brain” organoids – each about the size of a housefly’s eye – from adult skin stem cells that had been “reprogrammed” to grow into neurons.
Do you think we should be looking harder for alternatives to animal models in medicine? Share your thoughts in the comments!

In compliance with the JTI standards
More: SWI swissinfo.ch certified by the Journalism Trust Initiative











You can find an overview of ongoing debates with our journalists here . Please join us!
If you want to start a conversation about a topic raised in this article or want to report factual errors, email us at english@swissinfo.ch.