
How vaccine technology, choice and supply work in Switzerland
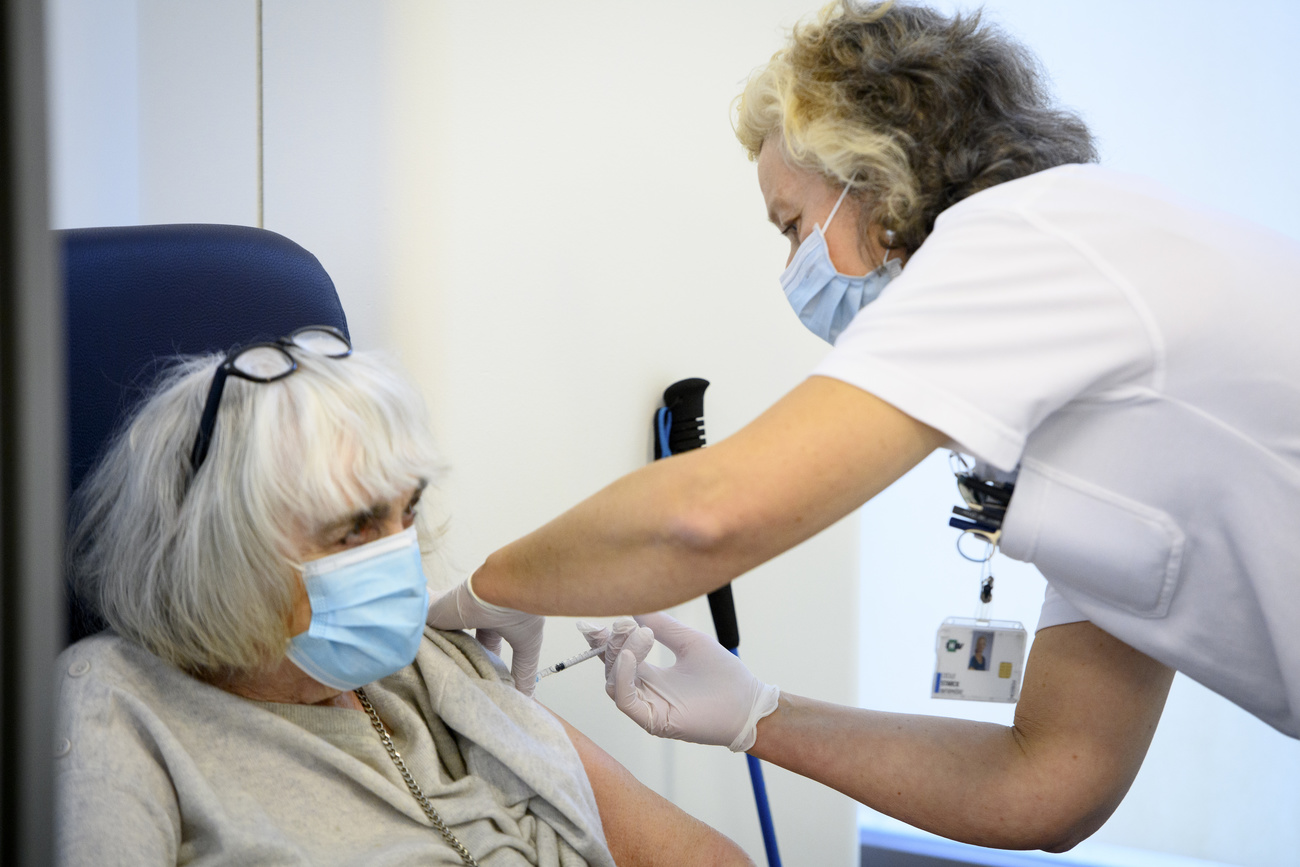
The Swiss Covid-19 vaccination campaign is up and running with two approved vaccines available to accelerate the fight against the virus, and others set to follow. How do the vaccine technologies work and compare, and can people choose which one they get?
So far, there are two vaccines available in Switzerland, those from Pfizer/BioNTech and Moderna. Demand far outstrips supply, but the recent approval of Moderna’s vaccine by regulator Swissmedic means that Switzerland “can now significantly increase our vaccination campaign”, according to Christoph Berger, president of the Federal Commission for Vaccinations.
The Swiss campaign has been hit by a temporary delay to the Pfizer/BioNTech Covid-19 vaccine in Europe. But this will not affect the country’s overall goal of inoculating all at-risk groups by the end of March, say Swiss health officials.
The Swiss government has set an ambitious target: to inoculate six million people or 70% of the population – on a voluntary basis – by summer, or up to 70,000 vaccine shots per day.
The Swiss government wants all elderly residents of old people’s homes to get a jab by the end of January. Anyone over 75 and the most vulnerable should get a shot by the end of February, followed by 70% of over-65s by the end of March. The rest of the population should then follow.
Vaccines will be offered in hospitals, clinics, regional vaccination centres, by mobile teams and in doctor’s practices. Pharmacies have also offered their services.
The two approved vaccines are very similar. Both use new messenger RNA (mRNA) technology, which contains instructions for human cells to make harmless spike proteins that mimic part of the coronavirus.
The instructions spur the immune system into action, prompting it to make antibodies and activate T-cells (a type of white blood cell) to fight the coronavirus. No actual live Covid-19 virus is contained in the vaccines and the mRNA, protected in oily bubbles of lipid nanoparticles, never enters the nucleus of the cell where our DNA is kept.
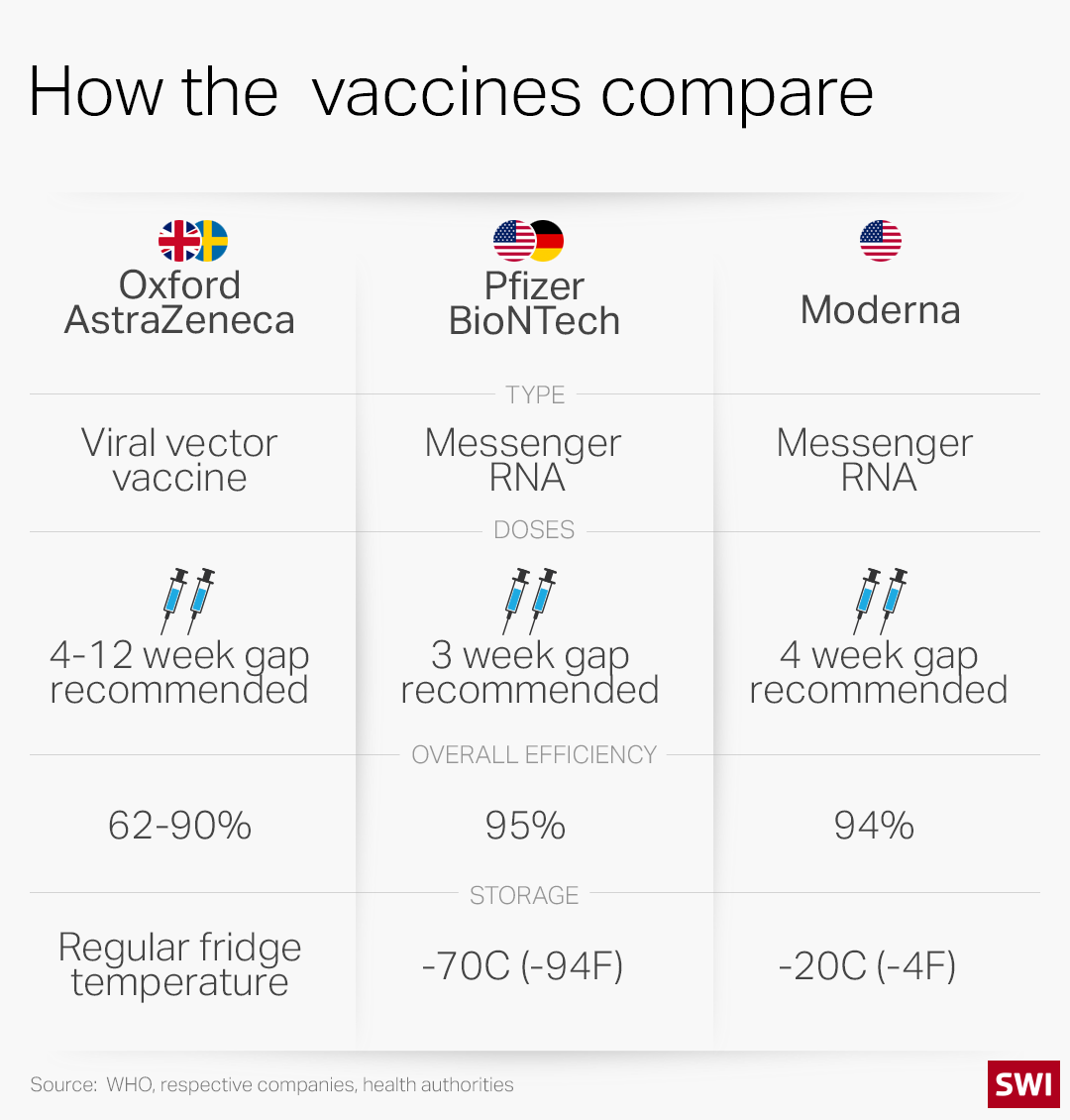
Trials have shown both mRNA vaccines to be highly effective (see graphic below). The main differences are how they are administered and stored.
The Pfizer/BioNTech vaccine must be kept at ultra-cold temperatures of -70 degrees Celsius (-94 Fahrenheit). Once thawed, it can only be refrigerated for five days. The vaccine requires a special shipping container packed with dry ice to keep it at the proper temperature.
Moderna’s vaccine can be stored at standard freezer temperatures of -20C (-4F) for up to six months. After it’s thawed, it can be kept in a refrigerator for up to 30 days.
“The storage and transportation [of the Moderna vaccine] are simpler, and its already in liquid form with ten doses per vial [compared to five for Pfizer/BioNTech that has to be carefully prepared],” explained Patrick Genoud, coordinator of Lausanne University Hospital (CHUV) vaccination centre.
“We can prepare the Moderna shots beforehand and transport them from one old people’s home to another with much greater flexibility.”

More
Switzerland’s stop-and-go march to vaccinate everyone by the summer
What other vaccines is Switzerland examining?
The next Covid-19 vaccine in the Swiss pipeline could be the one developed by the University of Oxford and the British-Swedish company AstraZeneca. A clinical trial revealed that the vaccine was 62-90% effective, depending on the initial dosage. Britain began vaccinating its population with Oxford/AstraZeneca Covid shots on January 4. It could win Swiss regulatory approval as early as this month, the NZZ newspaper reported last weekend.
The AstraZeneca shot is a “viral vector vaccine” and uses a different well-established technology. Researchers have taken genes for the spike protein on the surface of the coronavirus and put them into a harmless version of a common cold virus (known as an adenovirus) from chimpanzees. It has been altered to look more like coronavirus – although it can’t cause illness. When the vaccine is injected, it prompts the immune system to start making antibodies and prepares it to attack any coronavirus infection.
As well as being much cheaper (see infobox below), the AstraZeneca shot doesn’t require deep freezing. It can be kept in a standard refrigerator for six months.
Vaccine prices have remained largely secret as each country and bloc negotiates with the pharma firms. In December Belgium’s Budget State Secretary Eva De Bleeker let things slip in a tweet detailing the price of each vaccine purchased by the EU and how much Belgium will spend. The tweet has since been deleted, but the Belgian news site HLNExternal link published a screenshot. It showed that the EU is spending the following per coronavirus vaccine: Oxford/AstraZeneca: €1.78 (CHF1.92); Johnson & Johnson, $8.50; Sanofi/GSK: €7.56; BioNTech/Pfizer: €12; CureVac: €10; Moderna: $18. It is unclear how much Switzerland is spending for vaccines, which are free for Swiss residents.
The fourth vaccine being reviewed by Swissmedic is from pharmaceutical company Janssen-Cilag, which is part of the Johnson & Johnson Group. The candidate, another viral vector vaccine similar to the Oxford/AstraZeneca shot, is still advancing through clinical trials. Moncef Slaoui, the chief scientific adviser to the US government programme to develop and distribute Covid-19 vaccines, said the vaccine is expected to show efficacy at or above 80%. The advantage of Johnson & Johnson’s vaccine is that it requires just a single dose, and it can be transported and stored at 2-8C for three months and will last for two years if kept at -20C.
If trials show the vaccine is effective, the company could apply for emergency authorisation from the US Food and Drug Administration in February. A senior EU official recently told Reuters that it could be available in Europe in April. Johnson & Johnson submitted an applicationExternal link to the Swiss regulator for its vaccine candidate Ad26.COV2.S in December.
Can people choose which vaccine they get?
Blaise Genton, head of infectious diseases at Lausanne’s university medical centre Unisanté, believes patients probably won’t be able to choose which vaccine they get as more are rolled out. In some special cases the decision will fall to the patient’s doctor.
“There will probably be different indications for specific vaccines, such as for people with severe allergies, for whom mRNA vaccines are inappropriate and who might benefit from the Oxford/AstraZeneca, for example,” he declared.
As well as allergies, age may also be a deciding factor. Pfizer can be used from 16 years onwards, while the others are from 18, for example.
Experts say there are no major differences between the approved vaccines in terms of the protection they offer .
“The two mRNA vaccines are very comparable; they are very similar in terms of effectiveness and tolerability,” said Berger. “So, it doesn’t matter whether a person gets one or the other vaccine.”
Virginie Masserey, who heads infectious diseases at the Federal Office of Public Health, agreed.
“It is assumed that vaccines will be equivalent in terms of efficacy and safety, so it is rather the availability of the vaccine that will be decisive and the citizen will not need to choose,” she said.
And some say choice may be a luxury that countries can’t afford, with new variants of Covid-19 emerging and the virus continuing to spread.
More
“I would say at this moment that every vaccine with relatively good efficacy – that means, at least 60 or 70% – should be put to use, when it has an appropriate safety profile,” said Thomas Klimkait, a University of Basel professor and researcher.
How safe are the new vaccines?
Drug regulator Swissmedic closely monitors the safety of new vaccines. While some countries have rushed to grant approval for vaccines, Switzerland has taken a more cautious approach (Pfizer was approved on December 1 in UK and on December 20 in Switzerland; Moderna was approved on December 19 in US and on January 12 in Switzerland). In December, Swissmedic said emergency approval processes being used by some countries to speed up the roll-out of Covid-19 vaccines were not a legal option in Switzerland.
So far, no significant concerns have been reported, but nothing is 100% safe. Swissmedic says the side effects documented most frequently for Moderna and Pfizer/BioNTech vaccines were “comparable with those after a flu vaccination” (fatigue, headache, chills and muscle pain.)
Steve Pascolo, an immunologist at University Hospital Zurich, has been working with mRNA vaccines for over 20 years. He is convinced of the technology’s safety and does not expect any long-term side effects.
“The mRNA is very stable in the test tube in the laboratory. In the body, however, it is broken down very quickly by enzymes. So, what is injected is broken down by the body in a few hours, so that after a day or two there are no more traces of it,” he told Swiss public television, SRF.
Genton is also relaxed about the different technologies. He said he would be willing to take “any of the three vaccines” (Pfizer, Moderna or AstraZeneca). He emphasised that the ultra-fast research process had not in any way been “botched” or simplified.
“I know the vector viral vaccine as I’m familiar with the Ebola vaccine and I’ve worked a lot with malaria vaccines,” he said. “We’ve used this technology for 15 years. The Oxford team has even tested it on infants, so we should be reassured.”
But some Swiss immunologists, who have been trying to develop Covid-19 vaccines themselves, have certain reservations about the new mRNA technology, the procedures and substances used or even the possible long-term consequences.
“We don’t know whether it will cause long-term problems say for one person out of 20,000 in the next three years,” Bern University immunologist Martin Bachmann told SRF.
Covid-19 vaccine scepticism among Swiss health professionals appears to mirror that for the flu vaccine. A survey of 4,000 people in canton Neuchâtel last December revealed that 75% of doctors said they would get a Covid-19 jab, but the number fell to 36% for nurses.


In compliance with the JTI standards
More: SWI swissinfo.ch certified by the Journalism Trust Initiative

























Join the conversation!