Swiss doctors limit remdesivir use as government looks to procure more
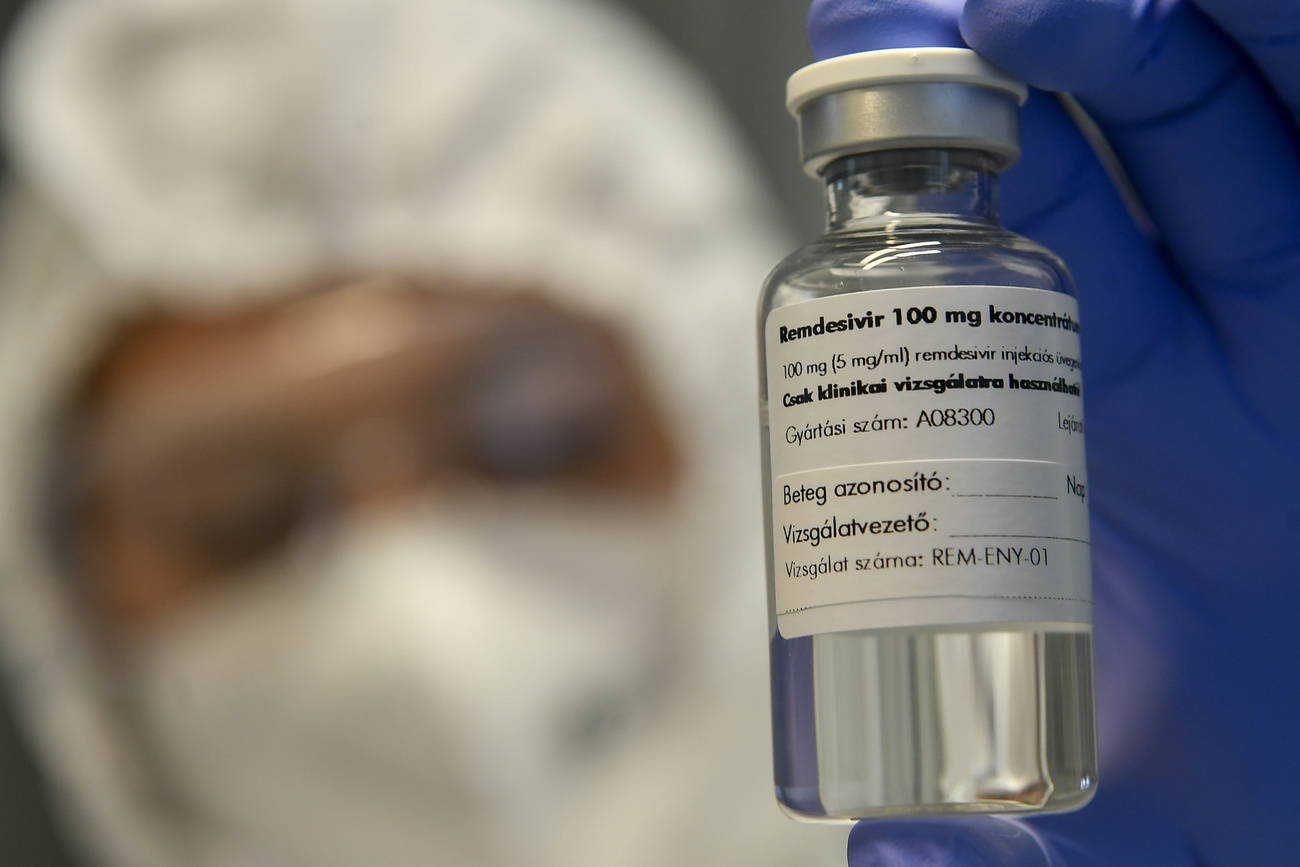
Swiss doctors are taking a cautious approach to the use of remdesivir for treating Covid-19 in the midst of emerging evidence from the World Health Organisation on the drug's ineffectiveness. Yet, the Swiss authorities moved ahead with an authorisation of the treatment and are making efforts to procure more of it.
The regulatory body, Swissmedic granted a temporary authorisation of remdesivir (under the brand name Veklury) on November 25, only five days after the World Health Organisation advisedExternal link against using the drug for treating hospitalised Covid-19 patients, regardless of disease severity. The Swiss government told SWI swissinfo.ch that it is also in talks with US firm Gilead, which developed the drug, to procure more doses of remdesivir for Covid-19 based on an agreement in August 2020.
The WHO based its recommendation on the interim resultsExternal link of the large-scale Solidarity trial, stating there is “currently no evidence that remdesivir improves survival and other outcomes in these patients”.
However other trials, including the ACTT-1 by the National Institute of Allergy and Infectious Diseases (NIAID) in the United States found that the drug shortened recovery time by five days.
“It is up to the treating physicians to prescribe the appropriate therapies on the basis of the product information approved by Swissmedic as part of the marketing authorisation and in the knowledge of the patient(s)”, a Swissmedic spokesperson told swissinfo.ch, explaining that its decision to authorise its use was based on the results of three trials including ACTT-1 by NIAID.
The physicians from the major university hospitals swissinfo.ch spoke to say they are limiting the use of remdesivir or not using it all, in line with the WHO updated guidelines. Alexandra Calmy, Oriol Manuel, and Hansjakob Furrer have been involved in the WHO-led Solidarity trial testing remdesivir.
“We only prescribe remdesivir in very selected cases. We have changed our attitude once the Solidarity trial results had been made available on October 16,” said Calmy, an associate physician at the University Hospital Geneva.
More
Sixteen Swiss hospitals take part in coronavirus global Solidarity trial
For his part, Manuel, said it was not being used where he practices at the Lausanne University Hospital. “My opinion is that the benefit is very minor. In Switzerland, it is restricted to very specific cases and mostly for treatment up to five days. The decision on whether to use the drug varies depending on the hospitals.”
In an articleExternal link published after the WHO recommendation in the German-language paper Neue Zürcher Zeitung, Peter Steiger, from the intensive care unit at Zurich University Hospital said that remdesivir is useful when used early. According to the article, the hospital is only using it in a targeted manner.
Similar views are shared by Hansjakob Furrer, head of the Department of Infectious Diseases, Bern University Hospital, Inselspital who added that his hospital has been very reluctant to use investigational drugs outside of study protocols.
“We have had only a very restricted use of remdesivir in very specific cases. The drug may be used only in high-risk cases and in the early phase of infection,” Furrer said.
He added that “just because WHO does not recommend it, does not mean that we will never use it because COVID-19 is a very heterogenous disease. There is no black or white. There could be use of the drug in very specific cases that further studies may reveal”.
Worth the price?
Given that some hospitals in Switzerland are following the WHO guidance, it remains to be seen how much remdesivir will ultimately be used and how valuable it will be for patients of Covid-19.
Neither the manufacturer, Gilead, nor the Swiss government indicated the quantities of remdesivir ordered, citing “contractual obligations” and on-going negotiations on the supply of the drug respectively. However, the Swiss government has confirmed that it is paying the price Gilead set for a vial (dose) – $390 (CHF380.50).
“The story of Gilead’s remdesivir strongly reminds us of Roche’s antiviral Tamiflu: an unjustifiably-high price given the low production costs and massive public funding, coupled with the fuelling of a governmental stockpiling for a drug that’s ultimately not worth it,” Patrick Durisch, Health Policy expert at the non-governmental organisation, Public Eye, told swissinfo.ch.
Remdesivir is a wide-spectrum antiviral therapy originally developedExternal link for hepatitis and was being studied against Ebola. Previous studies had shown that it had some effect with other coronaviruses (SARS and MERS) but it had never been approved by major regulators for use against any condition before Covid-19.
In the panic of the first wave of the pandemic, many governments used emergency powers to allow hospitals to use the drug even without conclusive evidence that it actually worked.
In Switzerland’s case, Swissmedic received an application for the temporary approval of remdesivir in June and initiated a fast-track procedure to authorise it. Under the Swiss Therapeutic Products Act, applications can be submitted even before drug development is fully completed. This allows Swissmedic to review available data while further clinical trial data are submitted at a later date.
Under the emergency measures adopted by the Federal Council, the Swiss government also allowed Swissmedic to procure essential medicinal products in the face of supply bottlenecks. In early July, Swissmedic allowed the temporary distributionExternal link of remdesivir while it reviewed its application for authorisation.
Gilead told swissinfo.ch that as per the agreement signed with the Swiss federal health office in August 2020, the quantity of remdesivir provided is based on the evolution of the local pandemic, which means that supply can be increased or decreased by the Swiss authorities according to the needs of patients.
In early November, Covid-19 positive cases surged to more than 10,000 new reported cases per day in Switzerland, which was far above the number of new daily infections during the first wave. The spread of the virus has since slowed but the situation remains “very worrying”, according to a recent statement by Health Minister Alain Berset.
This article is part of the series #BehindThePledge, a cross border investigation into the money trail of pandemic related medicines and vaccines. Journalismfund and IJ4EU awarded this project with grants. Ludovica Jona (Italy), Lise Barnéoud (France), Lucien Hordijk (The Netherlands), Hristio Boytchev (Germany) and Staffan Dahllöf (Denmark) contributed to the reporting of this story.
Priti Patnaik is the founding editor of Geneva Health FilesExternal link, a journalistic initiative that tracks the governance of global health, reporting on power and politics in international health policy.

In compliance with the JTI standards
More: SWI swissinfo.ch certified by the Journalism Trust Initiative








You can find an overview of ongoing debates with our journalists here . Please join us!
If you want to start a conversation about a topic raised in this article or want to report factual errors, email us at english@swissinfo.ch.