The end of affordable medicine
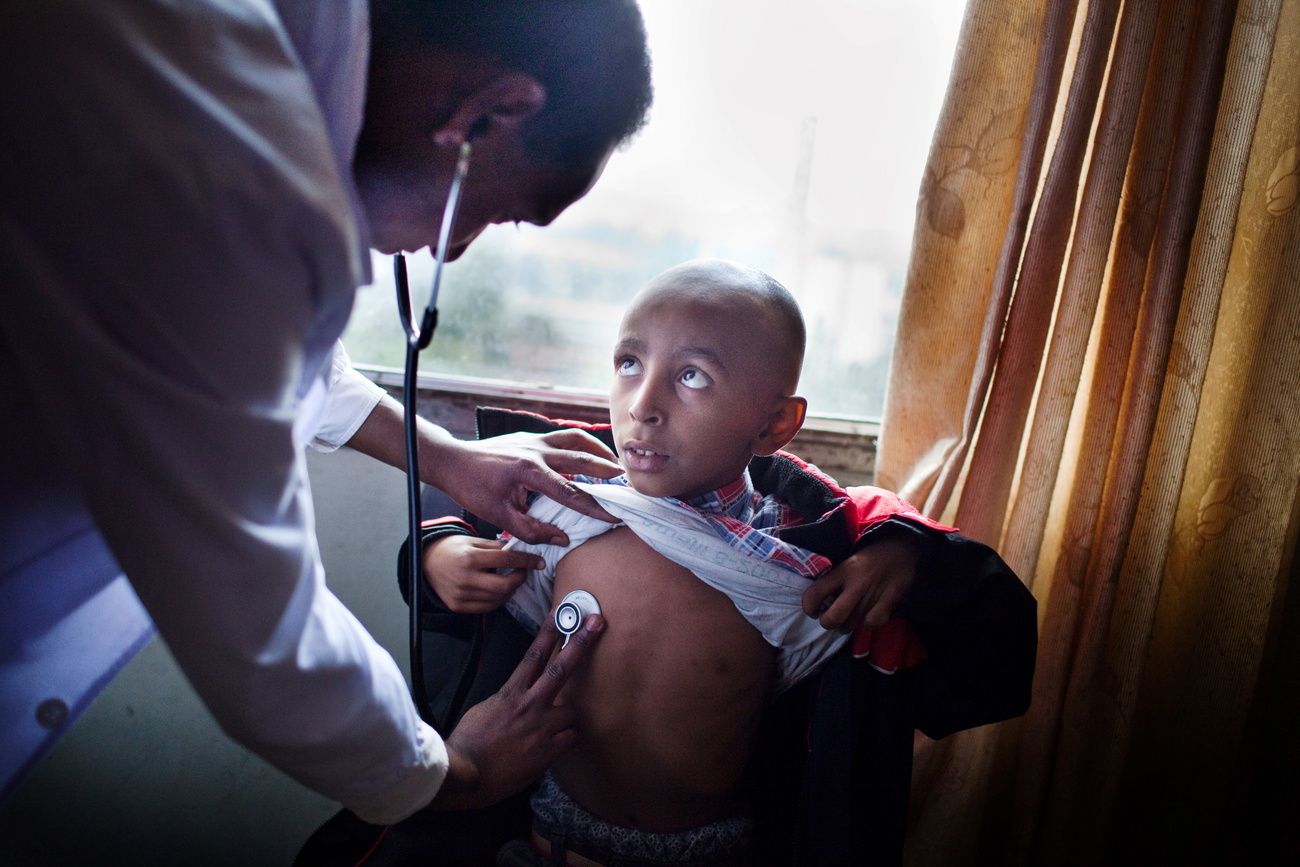
Pharmaceutical companies are close to a cure for many cancers and genetic diseases, but will the hefty price tags of the treatments make them unaffordable for most of the world?
Ten years ago, a team of cancer researchers at the University of Pennsylvania created the nearest thing to a miracle.
They removed white blood cells, an essential part of the immune system, from a 6-year-old girl with acute lymphoblastic leukemia – the most common childhood cancer. They reprogrammed the cells to give them cancer-fighting powers and injected them back into the girl, who had already relapsed twice after chemotherapy. Two weeks later, after waking from a coma, she was declared cancer-free.
This is part of a series of stories on the dilemmas facing governments, hospitals, and patients over the affordability of new, expensive treatments for cancer and other life-threatening genetic diseases. You can find all of the stories in the series here.
The treatment that enabled this near miracle was tisagenlecleucel, an immunotherapy that Swiss firm Novartis helped develop and now sells under the name Kymriah. It was the first CAR-T (Chimeric antigen receptor) therapy approved by US regulators, in 2017, and signaled the start of a new era in cancer cures. But even as doctors and patients celebrated the medical breakthrough, health insurers were bracing themselves for the financial fallout. The price tag Novartis put on a single infusion was a whopping $475,000.
Cancer drugs costing $200,000 (CHF200,000) or more a year aren’t unusual. The number of new therapies and their price tags have skyrocketed over the last few years, a trend that’s likely to continue as big pharma companies including Novartis prioritise innovative, “high-value medicines” with multi-billion-dollar sales potential based on the expectation that their life-changing, and even life-giving, properties will drive demand despite the cost.
But rising prices are weighing on health systems and patients around the world, and the impact is more profound in countries with limited resources, where a single drug can wipe out entire health budgets and where insurance coverage is still in its infancy, forcing patients to pay themselves.
“If I buy an expensive drug for one patient, I’ve almost depleted my entire budget and can’t purchase other drugs. What should I do when I know the drug will save the patient?” Gavin Orangi, an oncology pharmacist who manages the cancer clinic in Makueni county in south-eastern Kenya, told SWI swissinfo.ch.
More
Higher life expectancy and lifestyle changes have led to a surgeExternal link in cancer cases in many developing countries, where survival rates significantly lag those in developed nations. Of the 10 million cancer deaths recorded in 2020, some 70% were in low- and middle-income countries, World Health Organization (WHO) dataExternal link show. While some 85% of children with cancer are cured in SwitzerlandExternal link, in AfricaExternal link the figure is only 20%.
Health equity experts warn that rising prices could push the gap even wider. “There is almost a two-tiered track,” said André Ilbawi, a US-trained oncology surgeon who heads cancer control at the WHO. “One is defined by incredible innovation that inspires hope and on the other, populations who continue to have very little – even pain management at the end of life is difficult. That’s a profound injustice.”
Innovation revolution
The deluge of new drugs – and their fancy price tags – is being driven by breakthroughs in how scientists understand the immune system’s disease-fighting power and the genetic makeup of illnesses once considered incurable.
In the 1990s, treatments like breast cancer drug trastuzumab, which Swiss firm Roche sells under the name Herceptin, and the leukemia drug imatinib, sold by Novartis as Gleevec, dramatically improved survival rates by targeting the gene or protein that causes cancer cells to grow uncontrollably.
Checkpoint inhibitors, a form of immunotherapy that unlocks the immune system’s cancer-fighting powers, are among the latest advances. Around 10 checkpoint inhibitorsExternal link have been approved by US or European authorities since 2011. Cell therapies like Kymriah have gone a step further by rebooting the immune system through genetic engineering. Some seven cell therapies have been approved, and another 300 clinical trialsExternal link are underway.
Scientific advances have allowed researchers to create genetic maps of tumours and identify individual patients or groups that are more likely to respond to specific treatments.
More
How can a drug cost $2.1 million?
“It’s not far off to say that in the future a significant fraction of patients will have their own unique treatment based on their genetic profile and immune response,” said Olivier Michielin, head of oncology at Geneva University Hospital.
New technologies like mRNA, used for Covid-19 vaccines, are raising the prospect of personalised cancer vaccinesExternal link while gene therapies are emerging that can replace a missing or non-functioning gene with a normal one. These are offering hope of a long-term cure for cancer and many other deadly, inherited diseases like sickle cell. More than 60 new gene therapies are anticipated by 2030 and some two-thirds of gene therapy clinical trialsExternal link target cancer.
These breakthroughs have led to a surge in new treatments. Some 215 oncology drugs were launched globally in the last 20 years, nearly half in the last five years according to IQVIA, a global healthcare analytics and research group.
But innovation comes at a cost. Last year, global spending on cancer medicines – by governments, health insurers and patients – rose by 12% to a record $185 billion and could reach $300 billion in 2026, IQVIA estimates in its 2022 Oncology Trends reportExternal link. In Switzerland, the government’s annual bill for cancer-fighting drugs increased by 54% from 2014 to 2019 to an annual CHF931 million, according to an analysis by Swiss public television RTS.
While rising cancer cases and medicine use explain some of this, price is an important factor. A 2018 WHO report on the cost of cancer drugs found that the rate of growth in spending greatly exceededExternal link that of new cases.
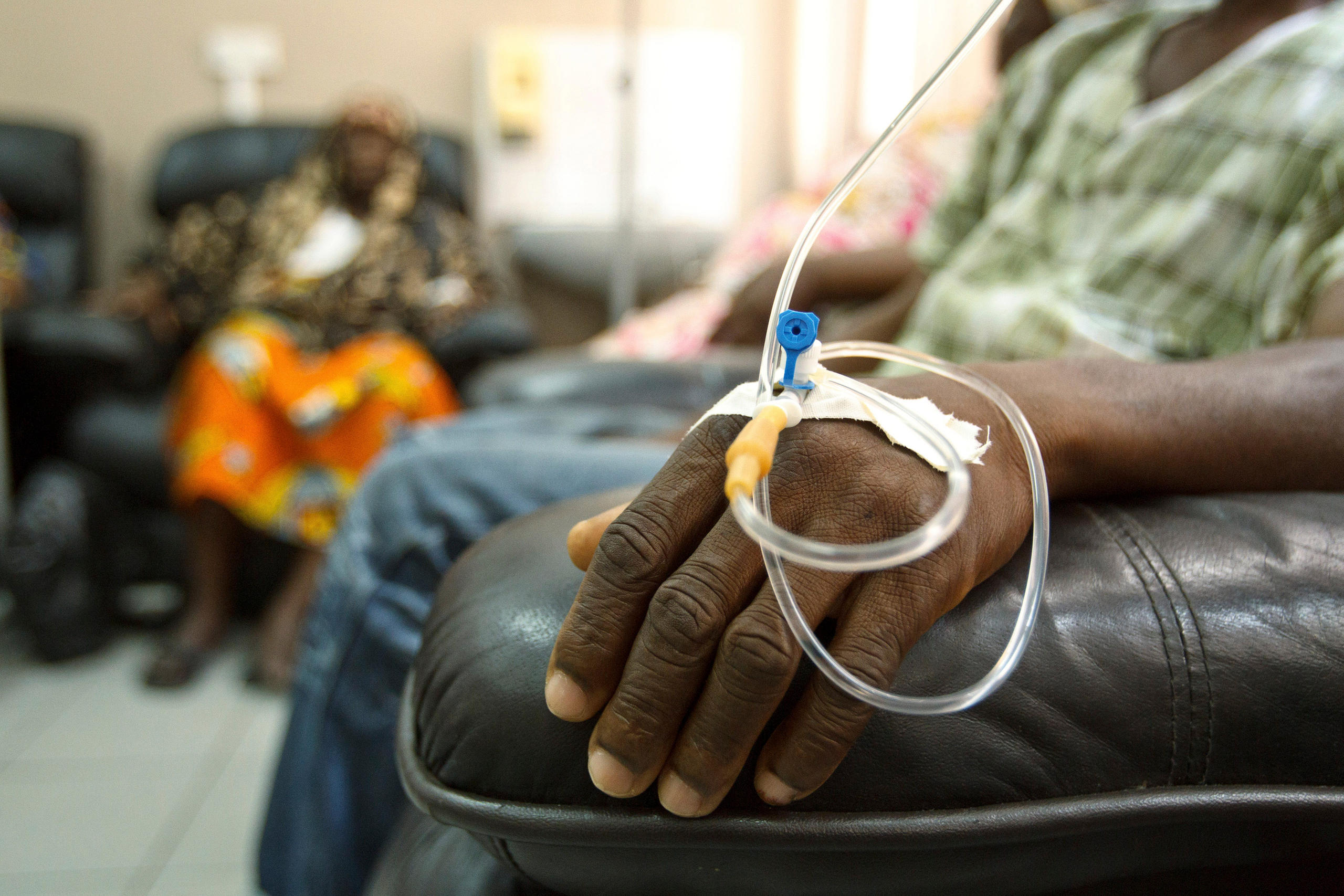
More
Why life-saving drugs aren’t saving lives in Kenya
It’s not difficult to see why pharma groups are so focused on oncology. From 2010 to 2019, revenue generatedExternal link from cancer drugs for the ten biggest pharma companies almost doubled from $52.8 billion to $103.5 billion, while revenue from non-oncology drugs fell by 19%.
In the US, over half of new cancer drugs approvedExternal link between 2009 and 2013 were priced at more than $100,000 per patient for a year of treatment. The median priceExternal link of new drugs rose from $1,932 in 1995-1999 to $14,950 in 2015-2019. Prices for gene therapies are expected to be even higher, with the first approved therapies already exceeding $2 million for one-time injections.
Pharmaceutical companies have justified high prices by pointing to studies that estimate it takes $90 million-$2.6 billion to develop a new drug and that companies need to factor in the high risk of failure. But the focus of their price narrative is now switching to the value medicinesExternal link bring to patients and society, including factors such as cost savings from fewer hospital stays.
Novartis’ CEO told ReutersExternal link in 2019 that “what’s often lost in the discussion is the remarkable impact of these medicines. These are true breakthroughs that come from a single infusion of a medicine that don’t require lifelong therapy.”
Drugmakers, who aren’t required to disclose how they set prices, are reluctant to get into specifics. “As a matter of principle, Roche doesn’t comment on price,” the company told SWI in a statement, adding that it uses the WHO’s definition of fair pricing which “balances the need for affordability for patients today and incentives for investing in R&D/innovation for the patients of tomorrow”.

More
High pharma margins squeeze health systems
Pressure for change is mounting from groups like the WHO, whose member states adopted a resolution in 2019 calling for more transparency. But so far, there’s been little progress.
The secrecy has drawn the ire of public health activists like Patrick Durisch, who leads health policy at Public Eye, a Swiss-based NGO focused on corporate accountability. “How do you know if you are getting a fair price if there is no transparency in the amount of money spent to develop the drug, and how much profit companies are making?” he asked. Profit margins on some cancer drugs that are still on-patent can range from 40%-90%, Public Eye has estimatedExternal link.
What’s at stake
The “value” of these new, expensive drugs is becoming a more important issue for governments and insurers who have to make tough decisions about whether and how much to pay for them. Some drugs are launched so quickly that there is limited clinical evidence of their benefits in terms of survival and quality of life.
Governments are starting to push back. In 2019, Norway rejected 22% of new medicines and treatments, mostly for cancer, because they were too expensive. Last year, following similar moves in the UK, the European Union and Japan started conducting their own evaluations of the cost-effectivenessExternal link of new drugs, forcing some companies to cut prices.
Acknowledging the resistance, some companies are proposing new payment models to ease the immediate financial burden. In Italy, for example, Novartis agreed to accept payment for Kymriah in three installmentsExternal link and only if certain patient outcomes are achieved.
But poorer nations have little leverage to negotiate prices. Countries with annual per capita income of under $30,000 account for only 14% of global oncology spendingExternal link, while 74% of spending comes from just seven countries – the US, UK, four European countries, and Japan.

While rich countries negotiate deals for Kymriah, Kenya is struggling to cover patient bills for trastuzumab, a treatment that’s been on the market for more than two decades.
“We can get immunotherapies,” said Naftali Busakhala, a physician who helped set up the cancer programme at Moi Teaching and Referral Hospital in Eldoret, Kenya. “The problem is cost. People can’t afford it.”
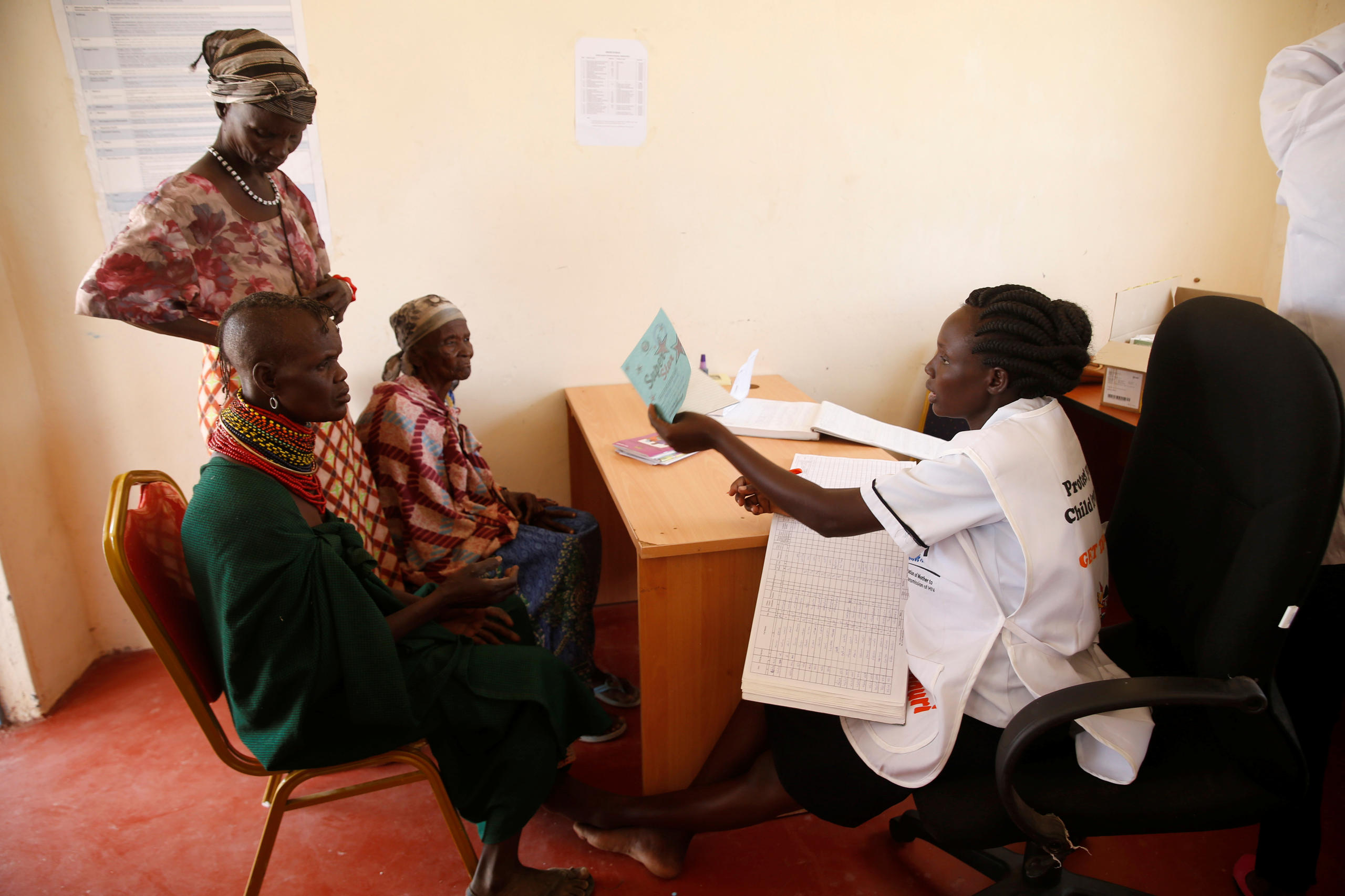
More
Can Novartis really make its medicines available to everyone?
Big pharma groups are increasingly turning to these emerging cancer markets though, and several, including Roche and Novartis, have committed to make their most innovative, expensive treatments accessible in low- and middle-income countries. “Fundamentally, innovations don’t matter if patients cannot access them,” Jackie Wambua, who heads government affairs at Roche’s East Africa office, told SWI.
But developing countries, facing a surge in cancer patients, also want the most advanced drugs now. “We need to make these medicines more affordable so that we start seeing cancer patients survive,” Mary Nyangasi, head of the cancer control programme at the Kenyan Ministry of Health, told SWI.
Additional reporting by Mercy Murugi. Photo editing by Helen James. Edited by Nerys Avery.
Why we chose to cover this story
There’s been incredible innovation in the treatment of cancer and other genetic diseases that is improving survival and quality of life for many people. However, these aren’t reaching most of the world. We wanted to understand why and what is being done to ensure that everyone has access to potentially life-saving treatment.
Finding sources
In all our reporting, we are vigilant in ensuring balanced reporting. This means all relevant facts and positions are considered when selecting sources to analyse a subject. In this case, we reached out to global health organisations with projects in Kenya for suggested experts, spoke to the largest pharmaceutical companies in Basel and in Kenya, and worked with a local journalist to identify government officials, hospitals, and patient organisations that are influencing the debate and create a space for patients to share their experiences. We traveled to Kenya for first-hand accounts of the topic, and to make our own observations of the issues.
If you want to know more about how we work, have a look here.
Get in touch
If you have any question regarding this topic that you’d like to have answered, or start a discussion with us and other readers, let us know via email: english@swissinfo.ch.

In compliance with the JTI standards
More: SWI swissinfo.ch certified by the Journalism Trust Initiative














You can find an overview of ongoing debates with our journalists here . Please join us!
If you want to start a conversation about a topic raised in this article or want to report factual errors, email us at english@swissinfo.ch.